当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recent advances in the synthetic chemistry of 1,5‐benzothiazepines: A minireview
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-20 , DOI: 10.1002/jhet.4062 Varsha Devi 1 , Gurpreet Singh 1 , Vikramdeep Monga 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-20 , DOI: 10.1002/jhet.4062 Varsha Devi 1 , Gurpreet Singh 1 , Vikramdeep Monga 1
Affiliation
|
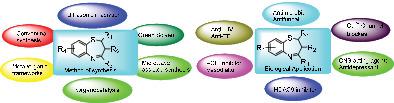
Benzothiazepine, a prominent “drug prejudice core”, is a heterocyclic moiety of immense importance due to its presence in a wide range of bioactive compounds. They act as a primary “biolinker” in diverse synthetic routes to obtain bioactive molecules and serve as important templates in synthetic and medicinal chemistry. They are known to possess a plethora of pharmacological activities, which include Ca2+ channel blockers, CNS acting agents, anti‐HIV, ACE inhibitors, antimicrobial, antifungal, anticancer. Their promising behaviour as drug molecules led the scientific community to develop novel, mild, green, and highly efficient synthetic routes for their synthesis. The conventional synthesis generally involved the condensation of chalcones with 2‐aminothiophenol in the presence of acid/base with high‐temperature heating, mostly resulting in poor yields or mixtures. However, recent trends are replacing these conditions with mild and green conditions through organocatalysis or other methodologies. In this review, an attempt has been made by authors to summarize (a) Recent developments in the synthetic strategies of 1,5‐benzoathiazepines and its derivatives (b) Conventional methods for the synthesis of 1,5‐benzothiazepines including progress in the green chemistry routes (c) Applications of various metals and organocatalysts to achieve the enantioselective synthesis of title compounds.
中文翻译:

1,5-苯并噻氮ia的合成化学的最新进展:综述
苯并噻氮平是一个突出的“药物偏见核心”,由于其广泛存在于多种生物活性化合物中,因此是极为重要的杂环部分。它们在各种合成途径中充当主要的“生物接头”,以获取生物活性分子,并在合成和药物化学中用作重要模板。已知它们具有大量的药理活性,包括Ca 2+通道阻滞剂,中枢神经系统作用剂,抗HIV,ACE抑制剂,抗微生物剂,抗真菌剂,抗癌药。它们作为药物分子的有前途的行为促使科学界开发出新颖,温和,绿色和高效的合成途径进行合成。常规合成通常涉及查耳酮与2-氨基苯硫酚在酸/碱存在下,高温加热下的缩合反应,主要导致收率或混合物不良。然而,最近的趋势是通过有机催化或其他方法用温和绿色条件代替这些条件。在这篇综述中,作者试图总结(a)1,5-苯并ath庚因及其衍生物的合成策略的最新进展(b)合成1,
更新日期:2020-09-08
中文翻译:

1,5-苯并噻氮ia的合成化学的最新进展:综述
苯并噻氮平是一个突出的“药物偏见核心”,由于其广泛存在于多种生物活性化合物中,因此是极为重要的杂环部分。它们在各种合成途径中充当主要的“生物接头”,以获取生物活性分子,并在合成和药物化学中用作重要模板。已知它们具有大量的药理活性,包括Ca 2+通道阻滞剂,中枢神经系统作用剂,抗HIV,ACE抑制剂,抗微生物剂,抗真菌剂,抗癌药。它们作为药物分子的有前途的行为促使科学界开发出新颖,温和,绿色和高效的合成途径进行合成。常规合成通常涉及查耳酮与2-氨基苯硫酚在酸/碱存在下,高温加热下的缩合反应,主要导致收率或混合物不良。然而,最近的趋势是通过有机催化或其他方法用温和绿色条件代替这些条件。在这篇综述中,作者试图总结(a)1,5-苯并ath庚因及其衍生物的合成策略的最新进展(b)合成1,



























 京公网安备 11010802027423号
京公网安备 11010802027423号