当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accelerated Polypeptide Synthesis via N-Carboxyanhydride Ring Opening Polymerization in Continuous Flow.
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2020-07-20 , DOI: 10.1002/marc.202000071 Jeroen Hendrik Vrijsen 1, 2 , Alicia Rasines Mazo 1 , Tanja Junkers 2, 3 , Greg Guanghua Qiao 1
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2020-07-20 , DOI: 10.1002/marc.202000071 Jeroen Hendrik Vrijsen 1, 2 , Alicia Rasines Mazo 1 , Tanja Junkers 2, 3 , Greg Guanghua Qiao 1
Affiliation
|
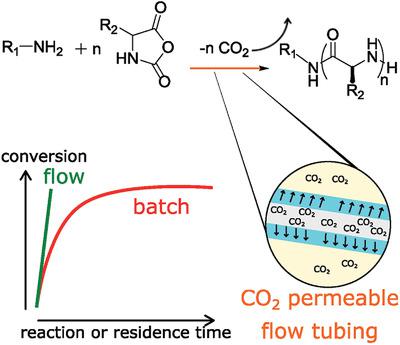
In nature, polypeptide‐based materials are ubiquitous, yet their synthetic production is hampered by high cost, limited scalability, and often stringent reaction conditions. Herein an elegant approach is presented for N‐carboxyanhydride ring opening polymerization (NCA ROP) of Nε‐benzyloxycarbonyl‐l‐lysine (ZLL) and γ‐benzyl‐l‐glutamate (BLG) NCA in continuous flow. The polymerization is initiated by primary amine initiators using N,N‐dimethylformamide (DMF) as solvent. Carrying out the reaction in a silicon microflow reactor speeds up the rate of ROP (92% conversion in 40 min in flow as opposed to 6 h in batch) due to highly efficient permeation of CO2 through the reactor tubing. The polymerization strategy provides a facile, scale‐up friendly alternative to traditional batch mode polymerization and has the capability of streamlining NCA ROP.
中文翻译:

通过N-羧甲基开环聚合在连续流中加速多肽合成。
本质上,基于多肽的材料无处不在,但其合成生产因高成本,有限的可扩展性以及通常严格的反应条件而受到阻碍。本文介绍了一种用于连续流动Nε-苄氧基羰基-1-赖氨酸(ZLL)和γ-苄基-1-谷氨酸(BLG)NCA的N-羧基酐开环聚合(NCA ROP)的优雅方法。聚合反应是使用N,N-二甲基甲酰胺(DMF)作为溶剂,由伯胺引发剂引发的。由于高效的CO 2渗透,在硅微流反应器中进行反应可加快ROP的速率(在40分钟的流动时间(分批处理6小时)中的转化率为92%)通过反应器管道。该聚合策略为传统的间歇式聚合提供了一种方便,按比例放大的替代方案,并且具有简化NCA ROP的能力。
更新日期:2020-09-22
中文翻译:

通过N-羧甲基开环聚合在连续流中加速多肽合成。
本质上,基于多肽的材料无处不在,但其合成生产因高成本,有限的可扩展性以及通常严格的反应条件而受到阻碍。本文介绍了一种用于连续流动Nε-苄氧基羰基-1-赖氨酸(ZLL)和γ-苄基-1-谷氨酸(BLG)NCA的N-羧基酐开环聚合(NCA ROP)的优雅方法。聚合反应是使用N,N-二甲基甲酰胺(DMF)作为溶剂,由伯胺引发剂引发的。由于高效的CO 2渗透,在硅微流反应器中进行反应可加快ROP的速率(在40分钟的流动时间(分批处理6小时)中的转化率为92%)通过反应器管道。该聚合策略为传统的间歇式聚合提供了一种方便,按比例放大的替代方案,并且具有简化NCA ROP的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号