当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantifying immunoregulation by autoantigen-specific T-regulatory type 1 cells in mice with simultaneous hepatic and extra-hepatic autoimmune disorders.
Immunology ( IF 6.4 ) Pub Date : 2020-07-20 , DOI: 10.1111/imm.13241 Hassan Jamaleddine 1 , Pere Santamaria 2, 3 , Anmar Khadra 1
Immunology ( IF 6.4 ) Pub Date : 2020-07-20 , DOI: 10.1111/imm.13241 Hassan Jamaleddine 1 , Pere Santamaria 2, 3 , Anmar Khadra 1
Affiliation
|
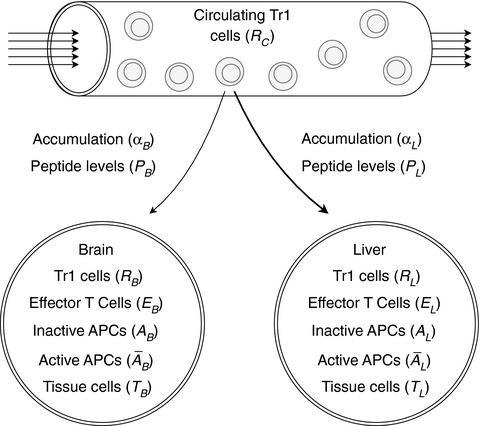
Nanoparticles (NPs) displaying autoimmune disease‐relevant peptide‐major histocompatibility complex class II molecules (pMHCII‐NPs) trigger cognate T‐regulatory type 1 (Tr1)‐cell formation and expansion, capable of reversing organ‐specific autoimmune responses. These pMHCII‐NPs that display epitopes from mitochondrial protein can blunt the progression of both autoimmune hepatitis (AIH) and experimental autoimmune encephalomyelitis (EAE) in mice carrying either disease. However, with co‐morbid mice having both diseases, these pMHCII‐NPs selectively treat AIH. In contrast, pMHCII‐NPs displaying central nervous system (CNS)‐specific epitopes can efficiently treat CNS autoimmunity, both in the absence and presence of AIH, without having any effects on the progression of the latter. Here, we develop a compartmentalized population model of T‐cells in co‐morbid mice to identify the mechanisms by which Tr1 cells mediate organ‐specific immunoregulation. We perform time‐series simulations and bifurcation analyses to study how varying physiological parameters, including local cognate antigenic load and rates of Tr1‐cell recruitment and retention, affect T‐cell allocation and Tr1‐mediated immunoregulation. Various regimes of behaviour, including ‘competitive autoimmunity’ where pMHCII‐NP‐treatment fails against both diseases, are identified and compared with experimental observations. Our results reveal that a transient delay in Tr1‐cell recruitment to the CNS, resulting from inflammation‐dependent Tr1‐cell allocation, accounts for the liver‐centric effects of AIH‐specific pMHCII‐NPs in co‐morbid mice as compared with mice exclusively having EAE. They also suggest that cognate autoantigen expression and local Tr1‐cell retention are key determinants of effective regulatory‐cell function. These results thus provide new insights into the rules that govern Tr1‐cell recruitment and their autoregulatory function.
中文翻译:

在同时患有肝脏和肝外自身免疫性疾病的小鼠中量化自身抗原特异性 T 调节 1 型细胞的免疫调节。
显示自身免疫疾病相关肽-主要组织相容性复合物 II 类分子 (pMHCII-NPs) 的纳米颗粒 (NPs) 触发同源 T 调节 1 (Tr1) 细胞形成和扩张,能够逆转器官特异性自身免疫反应。这些显示线粒体蛋白表位的 pMHCII-NPs 可以减缓携带任何一种疾病的小鼠的自身免疫性肝炎 (AIH) 和实验性自身免疫性脑脊髓炎 (EAE) 的进展。然而,对于患有这两种疾病的共病小鼠,这些 pMHCII-NPs 选择性地治疗 AIH。相比之下,显示中枢神经系统 (CNS) 特异性表位的 pMHCII-NPs 可以有效治疗 CNS 自身免疫,无论是否存在 AIH,对后者的进展没有任何影响。这里,我们在共病小鼠中开发了一个分区的 T 细胞群模型,以确定 Tr1 细胞介导器官特异性免疫调节的机制。我们进行时间序列模拟和分叉分析,以研究不同的生理参数,包括局部同源抗原负荷和 Tr1 细胞募集和保留率,如何影响 T 细胞分配和 Tr1 介导的免疫调节。确定了各种行为方式,包括“竞争性自身免疫”,其中 pMHCII-NP 治疗对两种疾病都失败,并与实验观察进行比较。我们的结果表明,由炎症依赖性 Tr1 细胞分配导致的 Tr1 细胞向 CNS 募集的短暂延迟,解释了与仅患有 EAE 的小鼠相比,AIH 特异性 pMHCII-NPs 在共病小鼠中的以肝脏为中心的作用。他们还表明同源自身抗原表达和局部 Tr1 细胞保留是有效调节细胞功能的关键决定因素。因此,这些结果为控制 Tr1 细胞募集及其自动调节功能的规则提供了新的见解。
更新日期:2020-07-20
中文翻译:

在同时患有肝脏和肝外自身免疫性疾病的小鼠中量化自身抗原特异性 T 调节 1 型细胞的免疫调节。
显示自身免疫疾病相关肽-主要组织相容性复合物 II 类分子 (pMHCII-NPs) 的纳米颗粒 (NPs) 触发同源 T 调节 1 (Tr1) 细胞形成和扩张,能够逆转器官特异性自身免疫反应。这些显示线粒体蛋白表位的 pMHCII-NPs 可以减缓携带任何一种疾病的小鼠的自身免疫性肝炎 (AIH) 和实验性自身免疫性脑脊髓炎 (EAE) 的进展。然而,对于患有这两种疾病的共病小鼠,这些 pMHCII-NPs 选择性地治疗 AIH。相比之下,显示中枢神经系统 (CNS) 特异性表位的 pMHCII-NPs 可以有效治疗 CNS 自身免疫,无论是否存在 AIH,对后者的进展没有任何影响。这里,我们在共病小鼠中开发了一个分区的 T 细胞群模型,以确定 Tr1 细胞介导器官特异性免疫调节的机制。我们进行时间序列模拟和分叉分析,以研究不同的生理参数,包括局部同源抗原负荷和 Tr1 细胞募集和保留率,如何影响 T 细胞分配和 Tr1 介导的免疫调节。确定了各种行为方式,包括“竞争性自身免疫”,其中 pMHCII-NP 治疗对两种疾病都失败,并与实验观察进行比较。我们的结果表明,由炎症依赖性 Tr1 细胞分配导致的 Tr1 细胞向 CNS 募集的短暂延迟,解释了与仅患有 EAE 的小鼠相比,AIH 特异性 pMHCII-NPs 在共病小鼠中的以肝脏为中心的作用。他们还表明同源自身抗原表达和局部 Tr1 细胞保留是有效调节细胞功能的关键决定因素。因此,这些结果为控制 Tr1 细胞募集及其自动调节功能的规则提供了新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号