当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intact or in Pieces? A Look at How Clinically Approved, Biodegradable Block Co-Polymers Affect Blood Components
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2020-07-19 , DOI: 10.1021/acsbiomaterials.0c00680 Lauren Maghak , Jessica Larsen
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2020-07-19 , DOI: 10.1021/acsbiomaterials.0c00680 Lauren Maghak , Jessica Larsen
|
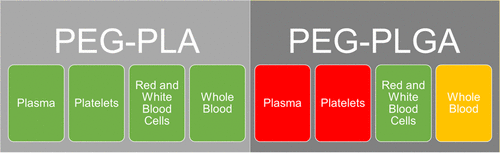
Polyethylene glycol-b-polylactic acid (PEG–PLA) and polyethylene glycol-b-poly(lactic-co-glycolic) acid) (PEG–PLGA) are two copolymers made up of three currently FDA approved polymers, used as drug delivery systems for specific indications. Here, the two were investigated to determine how they affected individual blood components and how they affected whole blood. Transmission and scanning electron microscopy imaging for each blood component and whole blood was completed with each amphiphilic copolymer for comparison. It was found that PEG–PLA does not disturb whole blood or its components, but PEG–PLGA harms platelets and plasma. These findings regarding PEG–PLGA show that this material is not compliant with all current FDA standards and could explain why clinical trials are facing complications.
中文翻译:

完整还是成块?临床批准的可生物降解的嵌段共聚合物如何影响血液成分
聚乙二醇- b -polylactic酸(PEG-PLA)和聚乙二醇- b -聚(乳酸-共-(乙醇酸)(PEG-PLGA)是由三种目前由FDA批准的聚合物组成的两种共聚物,用作特定适应症的药物输送系统。在这里,对这两者进行了调查,以确定它们如何影响单个血液成分以及如何影响全血。用每种两亲共聚物完成每种血液成分和全血的透射和扫描电子显微镜成像,以进行比较。已发现PEG-PLA不会干扰全血或其成分,但是PEG-PLGA会损害血小板和血浆。这些关于PEG-PLGA的发现表明,该材料不符合所有当前的FDA标准,并且可以解释为什么临床试验面临并发症。
更新日期:2020-09-14
中文翻译:

完整还是成块?临床批准的可生物降解的嵌段共聚合物如何影响血液成分
聚乙二醇- b -polylactic酸(PEG-PLA)和聚乙二醇- b -聚(乳酸-共-(乙醇酸)(PEG-PLGA)是由三种目前由FDA批准的聚合物组成的两种共聚物,用作特定适应症的药物输送系统。在这里,对这两者进行了调查,以确定它们如何影响单个血液成分以及如何影响全血。用每种两亲共聚物完成每种血液成分和全血的透射和扫描电子显微镜成像,以进行比较。已发现PEG-PLA不会干扰全血或其成分,但是PEG-PLGA会损害血小板和血浆。这些关于PEG-PLGA的发现表明,该材料不符合所有当前的FDA标准,并且可以解释为什么临床试验面临并发症。


























 京公网安备 11010802027423号
京公网安备 11010802027423号