当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Endocytosis‐Enabled Construction of Silica Nanochannels Crossing Living Cell Membrane for Transmembrane Drug Transport
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2020-07-19 , DOI: 10.1002/adfm.202002761 Xi Pan 1, 2 , Dandan Xu 1, 2 , Xiuzhen Tang 3 , Ning Liu 4 , Yongqiang You 1, 2 , Xianqiao Wang 4 , Xiaohui Yan 3 , Xing Ma 1, 2 , Xiaoyuan Chen 5
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2020-07-19 , DOI: 10.1002/adfm.202002761 Xi Pan 1, 2 , Dandan Xu 1, 2 , Xiuzhen Tang 3 , Ning Liu 4 , Yongqiang You 1, 2 , Xianqiao Wang 4 , Xiaohui Yan 3 , Xing Ma 1, 2 , Xiaoyuan Chen 5
Affiliation
|
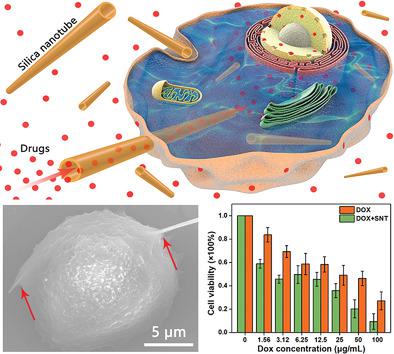
Artificial transmembrane channel (ATC) analogs are developed for overcoming biological membrane barriers and realizing transmembrane drug delivery, which are mostly studied within artificial lipid bilayers and thus lacked enough stability in practical applications on living cells. Here, natural endocytosis of silica‐based 1D nanomaterials (nanowires) with an ultrahigh aspect ratio is investigated. Enlightened by partially endocytosed ultralong silica nanowires, ATC that can penetrate living cell membranes for transmembrane transportation of small drug molecules is creatively constructed, resulting in enhanced drug delivery efficacy and decreased the half maximal inhibitory concentration. For the first time, an in‐depth study of the cellular uptake of 1D nanomaterials with ultrahigh aspect ratios (from 10 to 120) into living cells is carried out. Through confocal laser scanning microscopy observation, the endocytosis process of ultralong nanowires, including full uptake of short nanowires and partial uptake of longer nanowires, is clarified. Theoretical simulation is performed to give a fundamental understanding on the endocytosis mechanism of ultralong 1D silica nanowires. The simulation results demonstrate the time‐dependent internalization dynamics of the nanowires, which agrees well with our experimental results. This work not only clarifies the cellular interaction between 1D nanomaterials and living cells, but also pioneers the use of natural endocytosis of 1D nanomaterials for constructing ATC.
中文翻译:

具有吞噬作用的二氧化硅纳米通道构建跨活细胞膜的跨膜药物转运通道。
人工跨膜通道(ATC)类似物的开发是为了克服生物膜屏障和实现跨膜药物传递,这主要是在人工脂质双层中进行的研究,因此在活细胞的实际应用中缺乏足够的稳定性。在这里,研究了具有超高长宽比的二氧化硅基一维纳米材料(纳米线)的自然内吞作用。通过部分内吞的超长二氧化硅纳米线的启发,可以创造性地构建可以穿透活细胞膜以跨膜运输小药物分子的ATC,从而提高了药物的递送效率并降低了半数最大抑制浓度。首次对具有超高纵横比(从10到120)的一维纳米材料在细胞中的细胞吸收进行了深入研究。通过共聚焦激光扫描显微镜观察,阐明了超长纳米线的胞吞过程,包括完全吸收短纳米线和部分吸收较长纳米线。进行理论模拟,以便对超长一维二氧化硅纳米线的内吞作用机理有基本的了解。仿真结果证明了纳米线随时间的内在动力学,这与我们的实验结果非常吻合。这项工作不仅阐明了一维纳米材料与活细胞之间的细胞相互作用,而且开创了利用一维纳米材料的天然内吞作用构建ATC的先驱。已澄清。进行理论模拟,以便对超长一维二氧化硅纳米线的内吞作用机理有基本的了解。仿真结果证明了纳米线的时间依赖性内在动力学,这与我们的实验结果非常吻合。这项工作不仅阐明了一维纳米材料与活细胞之间的细胞相互作用,而且开创了利用一维纳米材料的天然内吞作用构建ATC的先驱。已澄清。进行理论模拟,以便对超长一维二氧化硅纳米线的内吞作用机理有基本的了解。仿真结果证明了纳米线随时间的内在动力学,这与我们的实验结果非常吻合。这项工作不仅阐明了一维纳米材料与活细胞之间的细胞相互作用,而且开创了利用一维纳米材料的天然内吞作用构建ATC的先驱。
更新日期:2020-09-18
中文翻译:

具有吞噬作用的二氧化硅纳米通道构建跨活细胞膜的跨膜药物转运通道。
人工跨膜通道(ATC)类似物的开发是为了克服生物膜屏障和实现跨膜药物传递,这主要是在人工脂质双层中进行的研究,因此在活细胞的实际应用中缺乏足够的稳定性。在这里,研究了具有超高长宽比的二氧化硅基一维纳米材料(纳米线)的自然内吞作用。通过部分内吞的超长二氧化硅纳米线的启发,可以创造性地构建可以穿透活细胞膜以跨膜运输小药物分子的ATC,从而提高了药物的递送效率并降低了半数最大抑制浓度。首次对具有超高纵横比(从10到120)的一维纳米材料在细胞中的细胞吸收进行了深入研究。通过共聚焦激光扫描显微镜观察,阐明了超长纳米线的胞吞过程,包括完全吸收短纳米线和部分吸收较长纳米线。进行理论模拟,以便对超长一维二氧化硅纳米线的内吞作用机理有基本的了解。仿真结果证明了纳米线随时间的内在动力学,这与我们的实验结果非常吻合。这项工作不仅阐明了一维纳米材料与活细胞之间的细胞相互作用,而且开创了利用一维纳米材料的天然内吞作用构建ATC的先驱。已澄清。进行理论模拟,以便对超长一维二氧化硅纳米线的内吞作用机理有基本的了解。仿真结果证明了纳米线的时间依赖性内在动力学,这与我们的实验结果非常吻合。这项工作不仅阐明了一维纳米材料与活细胞之间的细胞相互作用,而且开创了利用一维纳米材料的天然内吞作用构建ATC的先驱。已澄清。进行理论模拟,以便对超长一维二氧化硅纳米线的内吞作用机理有基本的了解。仿真结果证明了纳米线随时间的内在动力学,这与我们的实验结果非常吻合。这项工作不仅阐明了一维纳米材料与活细胞之间的细胞相互作用,而且开创了利用一维纳米材料的天然内吞作用构建ATC的先驱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号