当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rutaecarpine dissolved in binary aqueous solutions of methanol, ethanol, isopropanol and acetone: Solubility determination, solute-solvent and solvent-solvent interactions and preferential solvation study
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106253 Congcong Li , Yanxun Li , Xiaoqiang Gao , Hekun Lv
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106253 Congcong Li , Yanxun Li , Xiaoqiang Gao , Hekun Lv
|
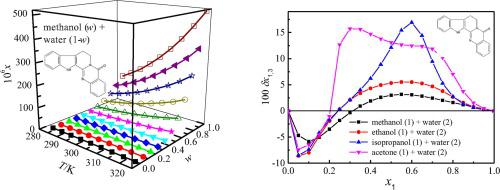
Abstract The main objective of this work was to report the mole fraction solubility of rutaecarpine in four binary solutions of methanol (1) + water (2), isopropanol (1) + water (2), acetone (1) + water (2) and ethanol (1) + water (2) acquired through the saturation shake-flask technique. All experiments were conducted at temperatures from 283.15 K to 323.15 K. The maximum solubility was observed in the pure solvent of methanol/ethanol/isopropanol/acetone for each solution. The achieved rutaecarpine solubility in mole fraction was mathematically described by two common co-solvency models, namely Jouyban-Acree model and van’t Hoff-Jouyban-Acree model. The calculated relative average deviations were no more than 6.78%; and root-mean-square deviations, no more than 23.69 × 10−6. The linear solvation energy relationships suggested by Kamlet and Taft was used to examine the effect of solvent descriptors on the behaviour of rutaecarpine solubility. The Hildebrand solubility parameter and dipolarity-polarizability were predominant contributors to solvent effect for the four mixtures studied. The local mole fractions of methanol (acetone, isopropanol or ethanol) and water nearby the solute rutaecarpine were quantitatively analyzed through an Inverse Kirkwood–Buff integrals method. For these mixtures within intermediate and methanol/ethanol/isopropanol/acetone-rich compositions, rutaecarpine was preferentially solvated by the methanol/ethanol/isopropanol/acetone; while within water-rich compositions, by the water.
中文翻译:

溶于甲醇、乙醇、异丙醇和丙酮的二元水溶液中的芸香碱:溶解度测定、溶质-溶剂和溶剂-溶剂相互作用和优先溶剂化研究
摘要 这项工作的主要目的是报告芸香碱在甲醇 (1) + 水 (2)、异丙醇 (1) + 水 (2)、丙酮 (1) + 水 (2) 的四种二元溶液中的摩尔分数溶解度和乙醇(1)+水(2)通过饱和摇瓶技术获得。所有实验均在 283.15 K 至 323.15 K 的温度下进行。在甲醇/乙醇/异丙醇/丙酮的纯溶剂中观察到每种溶液的最大溶解度。以摩尔分数计所达到的芸香果树碱溶解度通过两种常见的共溶解模型进行数学描述,即 Jouyban-Acree 模型和 van't Hoff-Jouyban-Acree 模型。计算出的相对平均偏差不超过6.78%;和均方根偏差,不超过 23.69 × 10−6。Kamlet 和 Taft 提出的线性溶剂化能关系用于检查溶剂描述符对芸香果树碱溶解度行为的影响。Hildebrand 溶解度参数和偶极-极化率是对所研究的四种混合物的溶剂效应的主要影响因素。甲醇(丙酮、异丙醇或乙醇)和溶质芸香碱附近的水的局部摩尔分数通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。Hildebrand 溶解度参数和偶极-极化率是所研究的四种混合物的溶剂效应的主要影响因素。甲醇(丙酮、异丙醇或乙醇)和溶质芸香碱附近的水的局部摩尔分数通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。Hildebrand 溶解度参数和偶极-极化率是所研究的四种混合物的溶剂效应的主要影响因素。甲醇(丙酮、异丙醇或乙醇)和溶质芸香碱附近的水的局部摩尔分数通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。异丙醇或乙醇)和溶质芸香碱附近的水通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。异丙醇或乙醇)和溶质芸香碱附近的水通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。
更新日期:2020-12-01
中文翻译:

溶于甲醇、乙醇、异丙醇和丙酮的二元水溶液中的芸香碱:溶解度测定、溶质-溶剂和溶剂-溶剂相互作用和优先溶剂化研究
摘要 这项工作的主要目的是报告芸香碱在甲醇 (1) + 水 (2)、异丙醇 (1) + 水 (2)、丙酮 (1) + 水 (2) 的四种二元溶液中的摩尔分数溶解度和乙醇(1)+水(2)通过饱和摇瓶技术获得。所有实验均在 283.15 K 至 323.15 K 的温度下进行。在甲醇/乙醇/异丙醇/丙酮的纯溶剂中观察到每种溶液的最大溶解度。以摩尔分数计所达到的芸香果树碱溶解度通过两种常见的共溶解模型进行数学描述,即 Jouyban-Acree 模型和 van't Hoff-Jouyban-Acree 模型。计算出的相对平均偏差不超过6.78%;和均方根偏差,不超过 23.69 × 10−6。Kamlet 和 Taft 提出的线性溶剂化能关系用于检查溶剂描述符对芸香果树碱溶解度行为的影响。Hildebrand 溶解度参数和偶极-极化率是对所研究的四种混合物的溶剂效应的主要影响因素。甲醇(丙酮、异丙醇或乙醇)和溶质芸香碱附近的水的局部摩尔分数通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。Hildebrand 溶解度参数和偶极-极化率是所研究的四种混合物的溶剂效应的主要影响因素。甲醇(丙酮、异丙醇或乙醇)和溶质芸香碱附近的水的局部摩尔分数通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。Hildebrand 溶解度参数和偶极-极化率是所研究的四种混合物的溶剂效应的主要影响因素。甲醇(丙酮、异丙醇或乙醇)和溶质芸香碱附近的水的局部摩尔分数通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。异丙醇或乙醇)和溶质芸香碱附近的水通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。异丙醇或乙醇)和溶质芸香碱附近的水通过逆柯克伍德-布夫积分法进行定量分析。对于中间体和富含甲醇/乙醇/异丙醇/丙酮的组合物中的这些混合物,芸香芸香碱优先被甲醇/乙醇/异丙醇/丙酮溶剂化;而在富含水的成分中,由水。



























 京公网安备 11010802027423号
京公网安备 11010802027423号