Cellular Signalling ( IF 4.8 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.cellsig.2020.109714 Avishek Halder 1 , Kamalendra Yadav 2 , Aanchal Aggarwal 2 , Nitin Singhal 2 , Rajat Sandhir 1
|
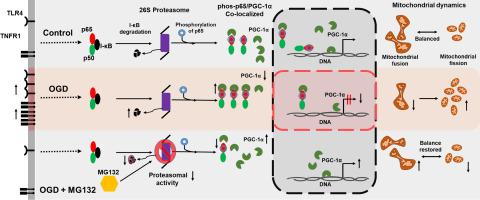
Astrocytes have emerged as active players in the innate immune response triggered by various types of insults. Recent literature suggests that mitochondria are key participants in innate immunity. The present study investigates the role of ischemia-induced innate immune response on p65/PGC-1α mediated mitochondrial dynamics in C6 astroglial cells. OGD conditions induced astroglial differentiation in C6 cells and increased the expression of hypoxia markers; HIF-1α, HO-1 and Cox4i2. OGD conditions resulted in induction of innate immune response in terms of expression of TNFR1 and TLR4 along with increase in IL-6 and TNF-α levels. OGD conditions resulted in decreased expression of I-κB with a concomitant increase in phos-p65 levels. The expression of PGC-1α, a key regulator of mitochondrial biogenesis, was also increased. Immunochemical staining suggested that phos-p65 and PGC-1α was co-localized. Studies on mitochondrial fusion (Mfn-1) and fission (DRP1) markers revealed shift toward fission. In addition, mitochondrial membrane potential decreased with increased DNA degradation and apoptosis confirming mitochondrial fission under OGD conditions. However, inhibition of phos-p65 by MG132 reduced the co-localization of phos-p65/ PGC-1α and significantly increased the Mfn-1 expression. The findings demonstrate the involvement of TNFR1 and TLR4 mediated immune response followed by interaction between phos-p65 and PGC-1α in promoting fission in C6 cells under hypoxic condition.
中文翻译:

缺氧葡萄糖后 TNFR1 和 TLR4 的激活促进 C6 星形胶质细胞中的线粒体裂变。
星形胶质细胞已成为由各种类型的侮辱引发的先天免疫反应的积极参与者。最近的文献表明,线粒体是先天免疫的关键参与者。本研究调查了缺血诱导的先天免疫反应对 C6 星形胶质细胞中 p65/PGC-1α 介导的线粒体动力学的作用。OGD 条件诱导 C6 细胞中的星形胶质细胞分化并增加缺氧标志物的表达;HIF-1α、HO-1 和 Cox4i2。OGD 条件导致在 TNFR1 和 TLR4 表达以及 IL-6 和 TNF-α 水平增加方面诱导先天免疫反应。OGD 条件导致 I-κB 表达降低,同时 phos-p65 水平增加。线粒体生物发生的关键调节因子 PGC-1α 的表达也增加。免疫化学染色表明 phos-p65 和 PGC-1α 是共定位的。对线粒体融合 (Mfn-1) 和裂变 (DRP1) 标记的研究揭示了向裂变的转变。此外,线粒体膜电位随着 DNA 降解和细胞凋亡的增加而降低,证实了 OGD 条件下的线粒体裂变。然而,MG132 对 phos-p65 的抑制降低了 phos-p65/PGC-1α 的共定位并显着增加了 Mfn-1 表达。这些发现证明了 TNFR1 和 TLR4 介导的免疫反应的参与,随后 phos-p65 和 PGC-1α 之间的相互作用促进了缺氧条件下 C6 细胞的分裂。线粒体膜电位随着 DNA 降解和细胞凋亡的增加而降低,证实了 OGD 条件下的线粒体裂变。然而,MG132 对 phos-p65 的抑制降低了 phos-p65/PGC-1α 的共定位并显着增加了 Mfn-1 表达。这些发现证明了 TNFR1 和 TLR4 介导的免疫反应的参与,随后 phos-p65 和 PGC-1α 之间的相互作用促进了缺氧条件下 C6 细胞的分裂。线粒体膜电位随着 DNA 降解和细胞凋亡的增加而降低,证实了 OGD 条件下的线粒体裂变。然而,MG132 对 phos-p65 的抑制降低了 phos-p65/PGC-1α 的共定位并显着增加了 Mfn-1 表达。这些发现证明了 TNFR1 和 TLR4 介导的免疫反应的参与,随后 phos-p65 和 PGC-1α 之间的相互作用促进了缺氧条件下 C6 细胞的分裂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号