Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.bbamcr.2020.118799 Jessica Canino 1 , Gianni Francesco Guidetti 2 , Luca Galgano 1 , Mauro Vismara 2 , Giampaolo Minetti 2 , Mauro Torti 2 , Ilaria Canobbio 2
|
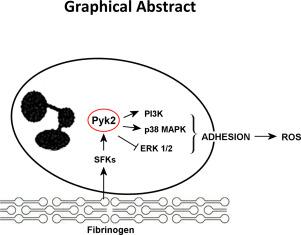
Neutrophils are first responders in infection and inflammation. They are able to roll, adhere and transmigrate through the endothelium to reach the site of infection, where they fight pathogens through secretion of granule contents, production of reactive oxygen species, extrusion of neutrophil extracellular traps, and phagocytosis. In this study we explored the role of the non-receptor focal adhesion kinase Pyk2 in neutrophil adhesion and activation. Using a specific Pyk2 pharmacological inhibitor, PF-4594755, as well as Pyk2-deficient murine neutrophils, we found that Pyk2 is activated upon integrin αMβ2-mediated neutrophil adhesion to fibrinogen. This process is triggered by Src family kinases-mediated phosphorylation and supported by Pyk2 autophosphorylation on Y402. In neutrophil adherent to fibrinogen, Pyk2 activates PI3K-dependent pathways promoting the phosphorylation of Akt and of its downstream effector GSK3. Pyk2 also dynamically regulates MAP kinases in fibrinogen-adherent neutrophils, as it stimulates p38MAPK but negatively regulates ERK1/2. Pharmacological inhibition of Pyk2 significantly prevented adhesion of human neutrophils to fibrinogen, and neutrophils from Pyk2-knockout mice showed a reduced ability to adhere compared to wildtype cells. Accordingly, neutrophil adhesion to fibrinogen was reduced upon inhibition of p38MAPK but potentiated by ERK1/2 inhibition. Neutrophil adherent to fibrinogen, but not to polylysine, were able to produce ROS upon lipopolysaccharide challenge and ROS production was completely suppressed upon inhibition of Pyk2. By contrast PMA-induced ROS production by neutrophil adherent to either fibrinogen or polylysine was independent from Pyk2. Altogether these results demonstrate that Pyk2 is an important effector in the coordinated puzzle regulating neutrophil adhesion and activation.
中文翻译:

富含脯氨酸的酪氨酸激酶Pyk2调节整联蛋白介导的嗜中性粒细胞粘附和活性氧的生成。
中性粒细胞是感染和炎症的第一反应者。它们能够滚动,粘附和迁移穿过内皮到达感染部位,在那里它们通过分泌颗粒成分,产生活性氧,挤压中性粒细胞胞外陷阱和吞噬作用来对抗病原体。在这项研究中,我们探索了非受体黏着斑激酶Pyk2在嗜中性粒细胞粘附和激活中的作用。使用特定的Pyk2药理抑制剂PF-4594755,以及缺乏Pyk2的鼠中性粒细胞,我们发现Pyk2在整合素αMβ2介导的中性粒细胞粘附到纤维蛋白原上时被激活。此过程由Src家族激酶介导的磷酸化触发,并由Y402上的Pyk2自磷酸化支持。在粘附于纤维蛋白原的中性粒细胞中,Pyk2激活PI3K依赖的途径,促进Akt及其下游效应物GSK3的磷酸化。Pyk2还可以动态调节纤维蛋白原粘附的中性粒细胞中的MAP激酶,因为它刺激p38MAPK,但对ERK1 / 2负调节。Pyk2的药理抑制作用显着阻止了人类嗜中性粒细胞粘附到纤维蛋白原上,与野生型细胞相比,Pyk2敲除小鼠的嗜中性粒细胞粘附能力降低。因此,中性粒细胞对纤维蛋白原的粘附在抑制p38MAPK时降低,但被ERK1 / 2抑制增强。粘附于纤维蛋白原而不粘附于聚赖氨酸的嗜中性粒细胞能够在脂多糖攻击后产生ROS,而抑制Pyk2则完全抑制了ROS的产生。相比之下,粘附于纤维蛋白原或聚赖氨酸的嗜中性粒细胞的PMA诱导的ROS产生与Pyk2无关。总而言之,这些结果表明Pyk2在调节嗜中性粒细胞的粘附和激活的协调难题中是重要的效应子。

























 京公网安备 11010802027423号
京公网安备 11010802027423号