Current Proteomics ( IF 0.8 ) Pub Date : 2020-09-30 , DOI: 10.2174/1570164617666191203111451 Farzaneh Afzali 1 , Parisa Ghahremanifard 2 , Mohammad Mehdi Ranjbar 3 , Mahdieh Salimi 4
|
Background: The tolerogenic homeostasis in Breast Cancer (BC) can be surpassed by rationally designed immune-encouraging constructs against tumor-specific antigens through immunoinformatics approach.
Objective: Availability of high throughput data providing the underlying concept of diseases and awarded computational simulations, lead to screening the potential medications and strategies in less time and cost. Despite the extensive effects of Placenta Specific 1 (PLAC1) in BC progression, immune tolerance, invasion, cell cycle regulation, and being a tumor-specific antigen the fundamental mechanisms and regulatory factors were not fully explored. It is also worth to design an immune response inducing construct to surpass the hurdles of traditional anti-cancer treatments.
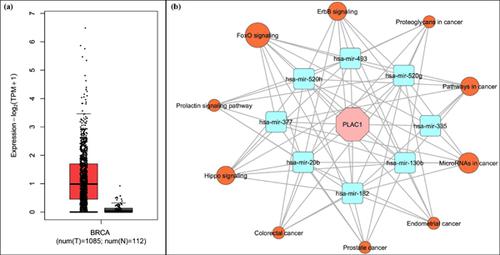
Methods and Result: The study was initiated by predicting and modelling the PLAC1 secondary and tertiary structures and then engineering the fusion pattern of PLAC1 derived immunodominant predicted CD8+ and B-cell epitopes to form a multi-epitope immunogenic construct. The construct was analyzed considering the physiochemical characterization, safety, antigenicity, post-translational modification, solubility, and intrinsically disordered regions. After modelling its tertiary structure, proteinprotein docking simulation was carried out to ensure the attachment of construct with Toll-Like Receptor 4 (TLR4) as an immune receptor. To guarantee the highest expression of the designed construct in E. coli k12 as an expressional host, the codon optimization and in-silico cloning were performed. The PLAC1 related miRNAs in BC were excavated and validated through TCGA BC miRNA-sequencing and databases; the common pathways then were introduced as other probable mechanisms of PLAC1 activity.
Conclusion: Regarding the obtained in-silico results, the designed anti-PLAC1 multi-epitope construct can probably trigger humoral and cellular immune responses and inflammatory cascades, therefore may have the potential of halting BC progression and invasion engaging predicted pathways.
中文翻译:

探索PLAC1的结构和潜在机制,以设计针对乳腺癌进展的衍生疫苗;硅内研究
背景:通过免疫信息学方法,针对肿瘤特异性抗原的合理设计的免疫鼓励构建体可以超越乳腺癌(BC)的耐受性稳态。
目的:高通量数据的可用性提供了疾病的基本概念并获得了奖励的计算模拟,从而可以在更短的时间和成本下筛选潜在的药物和策略。尽管胎盘特异性1(PLAC1)在BC进程,免疫耐受,侵袭,细胞周期调控以及作为肿瘤特异性抗原方面具有广泛的影响,但尚未充分探讨其基本机制和调控因素。还值得设计一种免疫应答诱导构建物,以超越传统抗癌治疗的障碍。
方法和结果:通过预测和建模PLAC1二级和三级结构,然后设计PLAC1衍生的免疫优势预测的CD8 +和B细胞表位的融合模式,以形成多表位免疫原性构建体,从而启动了这项研究。考虑到物理化学特征,安全性,抗原性,翻译后修饰,溶解度和固有无序区,对构建体进行了分析。在模拟其三级结构后,进行蛋白对接模拟以确保构建体与Toll样受体4(TLR4)作为免疫受体的连接。为确保设计的构建体在大肠杆菌k12中作为表达宿主的最高表达,密码子优化和计算机模拟进行克隆。通过TCGA BC miRNA测序和数据库对BC中与PLAC1相关的miRNA进行了挖掘和验证。然后介绍了常见途径,作为PLAC1活性的其他可能机制。
结论:关于获得的计算机模拟结果,设计的抗PLAC1多表位构建体可能会触发体液和细胞免疫反应以及炎症级联反应,因此可能具有阻止BC进程和参与预期途径的侵袭的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号