Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-07-17 , DOI: 10.1016/j.jinorgbio.2020.111178 Odra Pinato 1 , Anna Benettazzo 1 , Lisa Dalla Via 1 , Nicholas P Farrell 2 , Claudia Sissi 3
|
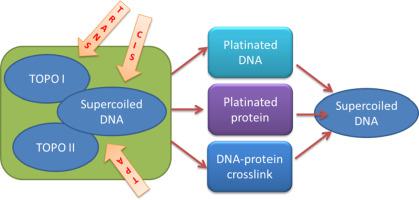
The clinical efficiency of Pt(II)-based drugs is founded on articulate mechanisms of action. Indeed it depends on a balanced combination of metal ion reactivity towards proteins and nucleic acids. Here we analysed the effect of two trans-platinum planar amines in comparison to cisplatin and transplatin on the DNA processivity by human topoisomerases I and IIα. Each tested metal complex produces DNA adducts with unique geometrical features and, consistently, they exert different effects on the activity of tested enzymes. Moreover, our results highlighted more subtle consequences on the enzymatic activity by the tested metal complexes which derive from a combination of preferential DNA or protein platination. Moreover, we observed that it is not possible to predict the overall output based only on the cis- vs trans- geometry of the tested metal complexes. This variable behaviour reflects the chemical reactivity profile of each single metal complex and can be usefully addressed to describe their different properties in the complex physiological environment.
中文翻译:

基于Pt(II)的复合物对人类拓扑异构酶松弛活性的调节。
基于Pt(II)的药物的临床效率基于明确的作用机制。实际上,这取决于金属离子对蛋白质和核酸的反应性的平衡组合。在这里,我们分析了与顺铂和反铂相比,两种反铂平面胺对人拓扑异构酶I和IIα的DNA合成能力的影响。每种经过测试的金属络合物均产生具有独特几何特征的DNA加合物,并且始终如一地,它们对测试酶的活性产生不同的影响。此外,我们的结果强调了被测金属配合物对酶活性的更微妙的影响,这些金属配合物源于优先DNA或蛋白质电镀的组合。此外,我们观察到不可能仅基于被测金属配合物的顺式-反式-几何结构。这种可变的行为反映了每种单一金属络合物的化学反应谱,可以有效地描述它们在复杂的生理环境中的不同性质。

























 京公网安备 11010802027423号
京公网安备 11010802027423号