当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photodecay of guaiacol is faster in ice, and even more rapid on ice, than in aqueous solution.
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-07-16 , DOI: 10.1039/d0em00242a Ted Hullar 1 , Fernanda C Bononi , Zekun Chen , Danielle Magadia , Oliver Palmer , Theo Tran , Dario Rocca , Oliviero Andreussi , Davide Donadio , Cort Anastasio
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-07-16 , DOI: 10.1039/d0em00242a Ted Hullar 1 , Fernanda C Bononi , Zekun Chen , Danielle Magadia , Oliver Palmer , Theo Tran , Dario Rocca , Oliviero Andreussi , Davide Donadio , Cort Anastasio
Affiliation
|
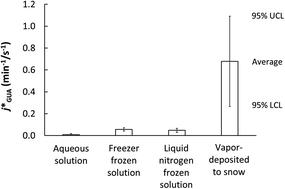
Snowpacks contain a wide variety of inorganic and organic compounds, including some that absorb sunlight and undergo direct photoreactions. How the rates of these reactions in, and on, ice compare to rates in water is unclear: some studies report similar rates, while others find faster rates in/on ice. Further complicating our understanding, there is conflicting evidence whether chemicals react more quickly at the air–ice interface compared to in liquid-like regions (LLRs) within the ice. To address these questions, we measured the photodegradation rate of guaiacol (2-methoxyphenol) in various sample types, including in solution, in ice, and at the air–ice interface of nature-identical snow. Compared to aqueous solution, we find modest rate constant enhancements (increases of 3- to 6-fold) in ice LLRs, and much larger enhancements (of 17- to 77-fold) at the air–ice interface of nature-identical snow. Our computational modeling suggests the absorption spectrum for guaiacol red-shifts and increases on ice surfaces, leading to more light absorption, but these changes explain only a small portion (roughly 2 to 9%) of the observed rate constant enhancements in/on ice. This indicates that increases in the quantum yield are primarily responsible for the increased photoreactivity of guaiacol on ice; relative to solution, our results suggest that the quantum yield is larger by a factor of roughly 3–6 in liquid-like regions and 12–40 at the air–ice interface.
中文翻译:

与水溶液相比,愈创木酚在冰中的光衰减更快,在冰上甚至更快。
积雪袋包含多种无机和有机化合物,包括一些吸收阳光并进行直接光反应的化合物。目前尚不清楚冰上和冰上这些反应的速率与水中的速率如何比较:一些研究报告的速率相似,而另一些研究发现在冰上或冰上的速率更快。进一步使我们的理解更加复杂的是,有证据表明,与冰中的液体状区域(LLR)相比,化学物质在气冰界面上的反应是否更快。为了解决这些问题,我们测量了各种样品类型中的愈创木酚(2-甲氧基苯酚)的光降解速率,包括溶液中,冰中以及与自然相同的雪的空气-冰界面。与水溶液相比,我们发现冰LLR中的速率常数适度增强(增加了3至6倍),并且在与自然相同的雪的空气-冰界面处进行了更大的增强(17到77倍)。我们的计算模型表明愈创木酚红移的吸收光谱在冰面上增加,导致更多的光吸收,但是这些变化仅解释了冰上/冰上观测到的速率常数增强的一小部分(大约2%至9%)。这表明量子产率的增加主要是由于愈创木酚在冰上的光反应性增加所致;相对于解决方案,我们的结果表明,在类似液体的区域中,量子产率大3-6倍,而在空气-冰界面处的量子产率则大12-40倍。导致更多的光吸收,但是这些变化仅解释了冰上/冰上观测到的速率常数增强的一小部分(大约2%至9%)。这表明量子产率的增加主要是由于愈创木酚在冰上的光反应性增加所致;相对于解决方案,我们的结果表明,在液体状区域中,量子产率大3-6倍,在空气-冰界面处,量子产率大12-40倍。导致更多的光吸收,但是这些变化仅解释了冰上/冰上观测到的速率常数增强的一小部分(大约2%至9%)。这表明量子产率的增加主要是由于愈创木酚在冰上的光反应性增加所致;相对于解决方案,我们的结果表明,在液体状区域中,量子产率大3-6倍,在空气-冰界面处,量子产率大12-40倍。
更新日期:2020-08-19
中文翻译:

与水溶液相比,愈创木酚在冰中的光衰减更快,在冰上甚至更快。
积雪袋包含多种无机和有机化合物,包括一些吸收阳光并进行直接光反应的化合物。目前尚不清楚冰上和冰上这些反应的速率与水中的速率如何比较:一些研究报告的速率相似,而另一些研究发现在冰上或冰上的速率更快。进一步使我们的理解更加复杂的是,有证据表明,与冰中的液体状区域(LLR)相比,化学物质在气冰界面上的反应是否更快。为了解决这些问题,我们测量了各种样品类型中的愈创木酚(2-甲氧基苯酚)的光降解速率,包括溶液中,冰中以及与自然相同的雪的空气-冰界面。与水溶液相比,我们发现冰LLR中的速率常数适度增强(增加了3至6倍),并且在与自然相同的雪的空气-冰界面处进行了更大的增强(17到77倍)。我们的计算模型表明愈创木酚红移的吸收光谱在冰面上增加,导致更多的光吸收,但是这些变化仅解释了冰上/冰上观测到的速率常数增强的一小部分(大约2%至9%)。这表明量子产率的增加主要是由于愈创木酚在冰上的光反应性增加所致;相对于解决方案,我们的结果表明,在类似液体的区域中,量子产率大3-6倍,而在空气-冰界面处的量子产率则大12-40倍。导致更多的光吸收,但是这些变化仅解释了冰上/冰上观测到的速率常数增强的一小部分(大约2%至9%)。这表明量子产率的增加主要是由于愈创木酚在冰上的光反应性增加所致;相对于解决方案,我们的结果表明,在液体状区域中,量子产率大3-6倍,在空气-冰界面处,量子产率大12-40倍。导致更多的光吸收,但是这些变化仅解释了冰上/冰上观测到的速率常数增强的一小部分(大约2%至9%)。这表明量子产率的增加主要是由于愈创木酚在冰上的光反应性增加所致;相对于解决方案,我们的结果表明,在液体状区域中,量子产率大3-6倍,在空气-冰界面处,量子产率大12-40倍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号