当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Thermostable Protein Matrix for Spectroscopic Analysis of Organic Semiconductors
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-07-16 , DOI: 10.1021/jacs.0c05477 George A Sutherland 1 , Daniel Polak 2 , David J K Swainsbury 1 , Shuangqing Wang 2 , Frank C Spano 3 , Dirk B Auman 4 , David G Bossanyi 2 , James P Pidgeon 2 , Andrew Hitchcock 1 , Andrew J Musser 2 , John E Anthony 5 , P Leslie Dutton 4 , Jenny Clark 2 , C Neil Hunter 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-07-16 , DOI: 10.1021/jacs.0c05477 George A Sutherland 1 , Daniel Polak 2 , David J K Swainsbury 1 , Shuangqing Wang 2 , Frank C Spano 3 , Dirk B Auman 4 , David G Bossanyi 2 , James P Pidgeon 2 , Andrew Hitchcock 1 , Andrew J Musser 2 , John E Anthony 5 , P Leslie Dutton 4 , Jenny Clark 2 , C Neil Hunter 1
Affiliation
|
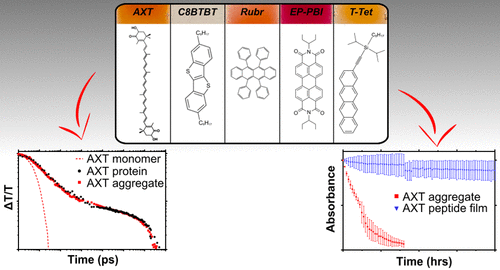
Advances in protein design and engineering have yielded peptide assemblies with enhanced and non-native functionalities. Here, various molecular organic semiconductors (OSCs), with known excitonic up- and down-conversion properties, are attached to a de novo-designed protein, conferring entirely novel functions on the peptide scaffolds. The protein-OSC complexes form similarly-sized, stable, water-soluble nanoparticles that are robust to cryogenic freezing and processing into the solid-state. The peptide matrix enables the formation of protein-OSC-trehalose glasses that fix the proteins in their folded states under oxygen-limited conditions. The encapsulation dramatically enhances the stability of protein-OSC complexes to photo-damage, increasing the lifetime of the chromophores from several hours to more than 10 weeks under constant illumination. Comparison of the photophysical properties of astaxanthin aggregates in mixed-solvent systems and proteins shows that the peptide environment does not alter the underlying electronic processes of the incorporated materials, exemplified here by singlet exciton fission followed by separation into weakly-bound, localized triplets. The adaptable protein-based approach lays the foundation for spectroscopic assessment of a broad range of molecular OSCs in aqueous solutions and the solid-state, circumventing the laborious procedure of identifying the experimental conditions necessary for aggregate generation or film formation. The non-native protein functions also raise the prospect of future bio-compatible devices where peptide assemblies could complex with native and non-native systems to generate novel functional materials.
中文翻译:

用于有机半导体光谱分析的热稳定蛋白质基质
蛋白质设计和工程的进步已经产生了具有增强和非天然功能的肽组装体。在这里,具有已知激子上转换和下转换特性的各种分子有机半导体 (OSC) 附着在从头设计的蛋白质上,赋予肽支架全新的功能。蛋白质-OSC 复合物形成大小相似、稳定的水溶性纳米颗粒,可耐受低温冷冻和加工成固态。肽基质能够形成蛋白质-OSC-海藻糖玻璃,在氧气受限的条件下将蛋白质固定在折叠状态。封装显着增强了蛋白质-OSC 复合物对光损伤的稳定性,将发色团的寿命从几个小时延长到 10 周以上。混合溶剂系统和蛋白质中虾青素聚集体的光物理性质的比较表明,肽环境不会改变掺入材料的潜在电子过程,这里的例子是单重激子裂变,然后分离成弱结合的局部三重态。适应性强的基于蛋白质的方法为光谱评估水溶液和固态中的各种分子 OSC 奠定了基础,避免了确定聚集体生成或成膜所需的实验条件的繁琐程序。非天然蛋白质功能也提高了未来生物相容性装置的前景,其中肽组件可以与天然和非天然系统复合以产生新的功能材料。
更新日期:2020-07-16
中文翻译:

用于有机半导体光谱分析的热稳定蛋白质基质
蛋白质设计和工程的进步已经产生了具有增强和非天然功能的肽组装体。在这里,具有已知激子上转换和下转换特性的各种分子有机半导体 (OSC) 附着在从头设计的蛋白质上,赋予肽支架全新的功能。蛋白质-OSC 复合物形成大小相似、稳定的水溶性纳米颗粒,可耐受低温冷冻和加工成固态。肽基质能够形成蛋白质-OSC-海藻糖玻璃,在氧气受限的条件下将蛋白质固定在折叠状态。封装显着增强了蛋白质-OSC 复合物对光损伤的稳定性,将发色团的寿命从几个小时延长到 10 周以上。混合溶剂系统和蛋白质中虾青素聚集体的光物理性质的比较表明,肽环境不会改变掺入材料的潜在电子过程,这里的例子是单重激子裂变,然后分离成弱结合的局部三重态。适应性强的基于蛋白质的方法为光谱评估水溶液和固态中的各种分子 OSC 奠定了基础,避免了确定聚集体生成或成膜所需的实验条件的繁琐程序。非天然蛋白质功能也提高了未来生物相容性装置的前景,其中肽组件可以与天然和非天然系统复合以产生新的功能材料。


























 京公网安备 11010802027423号
京公网安备 11010802027423号