当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radical-Mediated Strategies for the Functionalization of Alkenes with Diazo Compounds
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-07-15 , DOI: 10.1021/jacs.0c05183 Yong-Liang Su 1 , Geng-Xin Liu 2 , Jun-Wen Liu 2 , Linh Tram 1 , Huang Qiu 2 , Michael P Doyle 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-07-15 , DOI: 10.1021/jacs.0c05183 Yong-Liang Su 1 , Geng-Xin Liu 2 , Jun-Wen Liu 2 , Linh Tram 1 , Huang Qiu 2 , Michael P Doyle 1
Affiliation
|
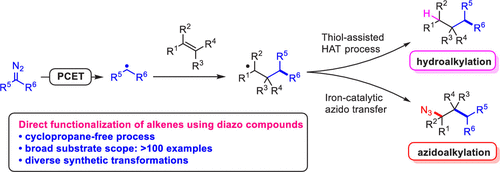
One of the most common reactions of diazo compounds with alkenes is cyclopropanation that occurs through metal carbene or free carbene intermediates. Alternative functionalization of alkenes with diazo compounds is limited, and methodology for the addi-tion of the elements of Z-CHR2 (with Z = H or heteroatom, and CHR2 originates from N2=CR2) across a carbon-carbon double bond has not been reported. Here we report a novel reaction of diazo compounds utilizing a radical-mediated addition strategy to achieve difunctionalization of diverse alkenes. Diazo compounds are transformed to carbon radicals with a photocatalyst or an iron catalyst through PCET processes. The carbon radical selectively adds to diverse alkenes delivering new carbon radical species, then forms products through hydroalkylation by thiol-assisted hydrogen atom transfer (HAT), or forms azidoalkylation products through an iron catalytic cycle. These two processes are highly complementary, proceed under mild reaction conditions, and show high functional group tolerance. Furthermore, both transformations are successfully performed on a gram-scale, and diverse γ-amino esters, γ-amino alcohols, and complex spirolactams are easily prepared with commercially available reagents. Mechanistic studies reveal the plausible pathways that link the two processes and explain the unique advantages of each.
中文翻译:

重氮化合物烯烃官能化的自由基介导策略
重氮化合物与烯烃最常见的反应之一是通过金属卡宾或游离卡宾中间体发生的环丙烷化反应。烯烃与重氮化合物的替代官能化是有限的,并且还没有通过碳碳双键添加 Z-CHR2 元素(Z = H 或杂原子,CHR2 源自 N2 = CR2)的方法报道。在这里,我们报告了一种利用自由基介导的加成策略实现多种烯烃双官能化的重氮化合物的新反应。重氮化合物在光催化剂或铁催化剂的作用下通过 PCET 过程转化为碳自由基。碳自由基选择性地添加到不同的烯烃中,提供新的碳自由基物种,然后通过硫醇辅助的氢原子转移 (HAT) 通过加氢烷基化形成产物,或通过铁催化循环形成叠氮烷基化产物。这两个过程是高度互补的,在温和的反应条件下进行,并表现出高度的官能团耐受性。此外,这两种转化都在克级规模上成功进行,并且可以使用市售试剂轻松制备各种 γ-氨基酯、γ-氨基醇和复杂的螺内酰胺。机理研究揭示了将这两个过程联系起来的合理途径,并解释了每个过程的独特优势。复杂的螺内酰胺很容易用市售试剂制备。机理研究揭示了将这两个过程联系起来的合理途径,并解释了每个过程的独特优势。复杂的螺内酰胺很容易用市售试剂制备。机理研究揭示了将这两个过程联系起来的合理途径,并解释了每个过程的独特优势。
更新日期:2020-07-15
中文翻译:

重氮化合物烯烃官能化的自由基介导策略
重氮化合物与烯烃最常见的反应之一是通过金属卡宾或游离卡宾中间体发生的环丙烷化反应。烯烃与重氮化合物的替代官能化是有限的,并且还没有通过碳碳双键添加 Z-CHR2 元素(Z = H 或杂原子,CHR2 源自 N2 = CR2)的方法报道。在这里,我们报告了一种利用自由基介导的加成策略实现多种烯烃双官能化的重氮化合物的新反应。重氮化合物在光催化剂或铁催化剂的作用下通过 PCET 过程转化为碳自由基。碳自由基选择性地添加到不同的烯烃中,提供新的碳自由基物种,然后通过硫醇辅助的氢原子转移 (HAT) 通过加氢烷基化形成产物,或通过铁催化循环形成叠氮烷基化产物。这两个过程是高度互补的,在温和的反应条件下进行,并表现出高度的官能团耐受性。此外,这两种转化都在克级规模上成功进行,并且可以使用市售试剂轻松制备各种 γ-氨基酯、γ-氨基醇和复杂的螺内酰胺。机理研究揭示了将这两个过程联系起来的合理途径,并解释了每个过程的独特优势。复杂的螺内酰胺很容易用市售试剂制备。机理研究揭示了将这两个过程联系起来的合理途径,并解释了每个过程的独特优势。复杂的螺内酰胺很容易用市售试剂制备。机理研究揭示了将这两个过程联系起来的合理途径,并解释了每个过程的独特优势。

























 京公网安备 11010802027423号
京公网安备 11010802027423号