Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid Gel Card Agglutination Assays for Serological Analysis Following SARS-CoV-2 Infection in Humans.
ACS Sensors ( IF 8.9 ) Pub Date : 2020-07-16 , DOI: 10.1021/acssensors.0c01050 Diana Alves 1, 2 , Rodrigo Curvello 1, 2 , Edward Henderson 1, 2, 3 , Vidhishri Kesarwani 1, 2, 3 , Julia A Walker 1, 2, 3, 4 , Samuel C Leguizamon 1, 2, 5, 6 , Heather McLiesh 1, 2 , Vikram Singh Raghuwanshi 1, 2 , Hajar Samadian 1, 2 , Erica M Wood 7, 8 , Zoe K McQuilten 7, 8 , Maryza Graham 9, 10, 11 , Megan Wieringa 9, 11 , Tony M Korman 9, 10, 12 , Timothy F Scott 1, 2, 5 , Mark M Banaszak Holl 1, 2 , Gil Garnier 1, 2 , Simon R Corrie 1, 2, 3, 4
ACS Sensors ( IF 8.9 ) Pub Date : 2020-07-16 , DOI: 10.1021/acssensors.0c01050 Diana Alves 1, 2 , Rodrigo Curvello 1, 2 , Edward Henderson 1, 2, 3 , Vidhishri Kesarwani 1, 2, 3 , Julia A Walker 1, 2, 3, 4 , Samuel C Leguizamon 1, 2, 5, 6 , Heather McLiesh 1, 2 , Vikram Singh Raghuwanshi 1, 2 , Hajar Samadian 1, 2 , Erica M Wood 7, 8 , Zoe K McQuilten 7, 8 , Maryza Graham 9, 10, 11 , Megan Wieringa 9, 11 , Tony M Korman 9, 10, 12 , Timothy F Scott 1, 2, 5 , Mark M Banaszak Holl 1, 2 , Gil Garnier 1, 2 , Simon R Corrie 1, 2, 3, 4
Affiliation
|
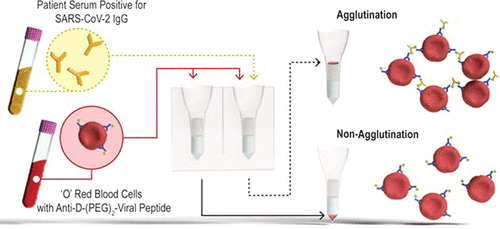
High-throughput and rapid serology assays to detect the antibody response specific to severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) in human blood samples are urgently required to improve our understanding of the effects of COVID-19 across the world. Short-term applications include rapid case identification and contact tracing to limit viral spread, while population screening to determine the extent of viral infection across communities is a longer-term need. Assays developed to address these needs should match the ASSURED criteria. We have identified agglutination tests based on the commonly employed blood typing methods as a viable option. These blood typing tests are employed in hospitals worldwide, are high-throughput, fast (10–30 min), and automated in most cases. Herein, we describe the application of agglutination assays to SARS-CoV-2 serology testing by combining column agglutination testing with peptide–antibody bioconjugates, which facilitate red cell cross-linking only in the presence of plasma containing antibodies against SARS-CoV-2. This simple, rapid, and easily scalable approach has immediate application in SARS-CoV-2 serological testing and is a useful platform for assay development beyond the COVID-19 pandemic.
中文翻译:

用于人类 SARS-CoV-2 感染后血清学分析的快速凝胶卡凝集测定。
迫切需要进行高通量快速血清学检测来检测人类血液样本中针对严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 的抗体反应,以加深我们对 COVID-19 在全球影响的了解。短期应用包括快速病例识别和接触者追踪,以限制病毒传播,而长期需要进行人口筛查以确定跨社区的病毒感染程度。为满足这些需求而开发的检测方法应符合 ASSURED 标准。我们已经确定基于常用血型方法的凝集试验是一种可行的选择。这些血型测试在世界各地的医院中使用,在大多数情况下是高通量、快速(10-30 分钟)且自动化的。在此,我们通过将柱凝集测试与肽-抗体生物缀合物相结合,描述了凝集测定在 SARS-CoV-2 血清学测试中的应用,肽-抗体生物缀合物仅在含有 SARS-CoV-2 抗体的血浆存在时才促进红细胞交联。这种简单、快速且易于扩展的方法可立即应用于 SARS-CoV-2 血清学检测,并且是 COVID-19 大流行之外的检测开发的有用平台。
更新日期:2020-08-28
中文翻译:

用于人类 SARS-CoV-2 感染后血清学分析的快速凝胶卡凝集测定。
迫切需要进行高通量快速血清学检测来检测人类血液样本中针对严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 的抗体反应,以加深我们对 COVID-19 在全球影响的了解。短期应用包括快速病例识别和接触者追踪,以限制病毒传播,而长期需要进行人口筛查以确定跨社区的病毒感染程度。为满足这些需求而开发的检测方法应符合 ASSURED 标准。我们已经确定基于常用血型方法的凝集试验是一种可行的选择。这些血型测试在世界各地的医院中使用,在大多数情况下是高通量、快速(10-30 分钟)且自动化的。在此,我们通过将柱凝集测试与肽-抗体生物缀合物相结合,描述了凝集测定在 SARS-CoV-2 血清学测试中的应用,肽-抗体生物缀合物仅在含有 SARS-CoV-2 抗体的血浆存在时才促进红细胞交联。这种简单、快速且易于扩展的方法可立即应用于 SARS-CoV-2 血清学检测,并且是 COVID-19 大流行之外的检测开发的有用平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号