当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement, modelling and molecular dynamics analysis for isobaric vapour-liquid equilibria of binary or ternary system (diethylamine, ethyl acetate, triethylamine)
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106251 Xia Yin , Chaochen Du , Zhiping Du , Wenbo Jiang , Yigang Ding , Hongmei Du , Zhiguo Yan , Lilei Zhang
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106251 Xia Yin , Chaochen Du , Zhiping Du , Wenbo Jiang , Yigang Ding , Hongmei Du , Zhiguo Yan , Lilei Zhang
|
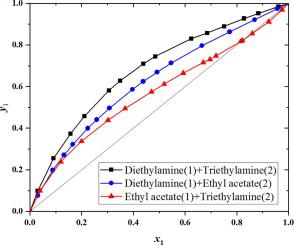
Abstract Diethylamine, ethyl acetate and triethylamine are usually applied to synthesize an important flame retardant of vinylphosphorus, which has high ability to retard flames, low toxic content, and good washing durability. The isobaric VLE data of (diethylamine + triethylamine, diethylamine + ethyl acetate) and (ethyl acetate + trimethylamine) system were determined at 101.3 kPa for industrial separation. A minimum azeotrope was discovered at x = 0.8217 in the (ethyl acetate + trimethylamine) system. Therefore, a series of experiments were designed to investigate the effect of diethylamine on the (ethyl acetate + trimethylamine) system and the results indicated that the azeotrope can be eliminated when the liquid mole fraction of diethylamine is greater than 0.4687. Furthermore, molecular dynamics analysis through GROMACS software and Multiwfn software was also used to study the interaction of these three materials in this work. The results show that the weak interaction force around ethyl acetate decreases after adding diethylamine, which means that the van der Waals effect and the electrostatic effect of ethyl acetate become smaller. This may be the main reason why the azeotropic point of the (ethyl acetate + trimethylamine) system disappears.
中文翻译:

二元或三元体系(二乙胺、乙酸乙酯、三乙胺)等压气液平衡的测量、建模和分子动力学分析
摘要 二乙胺、乙酸乙酯和三乙胺常用于合成重要的乙烯基磷阻燃剂,具有阻燃能力强、毒性低、耐洗性好等特点。(二乙胺+三乙胺、二乙胺+乙酸乙酯)和(乙酸乙酯+三甲胺)体系的等压VLE数据在101.3 kPa下测定,用于工业分离。在(乙酸乙酯 + 三甲胺)系统中,在 x = 0.8217 处发现了最小共沸物。因此,设计了一系列实验来研究二乙胺对(乙酸乙酯+三甲胺)体系的影响,结果表明,当二乙胺的液体摩尔分数大于0.4687时,可以消除共沸物。此外,通过 GROMACS 软件和 Multiwfn 软件的分子动力学分析也用于研究这三种材料的相互作用。结果表明,加入二乙胺后,乙酸乙酯周围的弱相互作用力减弱,表明乙酸乙酯的范德华效应和静电效应变小。这可能是(乙酸乙酯+三甲胺)体系共沸点消失的主要原因。
更新日期:2020-12-01
中文翻译:

二元或三元体系(二乙胺、乙酸乙酯、三乙胺)等压气液平衡的测量、建模和分子动力学分析
摘要 二乙胺、乙酸乙酯和三乙胺常用于合成重要的乙烯基磷阻燃剂,具有阻燃能力强、毒性低、耐洗性好等特点。(二乙胺+三乙胺、二乙胺+乙酸乙酯)和(乙酸乙酯+三甲胺)体系的等压VLE数据在101.3 kPa下测定,用于工业分离。在(乙酸乙酯 + 三甲胺)系统中,在 x = 0.8217 处发现了最小共沸物。因此,设计了一系列实验来研究二乙胺对(乙酸乙酯+三甲胺)体系的影响,结果表明,当二乙胺的液体摩尔分数大于0.4687时,可以消除共沸物。此外,通过 GROMACS 软件和 Multiwfn 软件的分子动力学分析也用于研究这三种材料的相互作用。结果表明,加入二乙胺后,乙酸乙酯周围的弱相互作用力减弱,表明乙酸乙酯的范德华效应和静电效应变小。这可能是(乙酸乙酯+三甲胺)体系共沸点消失的主要原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号