Journal of Structural Biology ( IF 3 ) Pub Date : 2020-07-16 , DOI: 10.1016/j.jsb.2020.107578 María Daniela Torres-Rodríguez 1 , Lilian González-Segura 1 , Rogelio Rodríguez-Sotres 1 , Javier Andrés Juárez-DíaZ 2 , Yuridia Cruz-Zamora 1 , Felipe Cruz-García 1
|
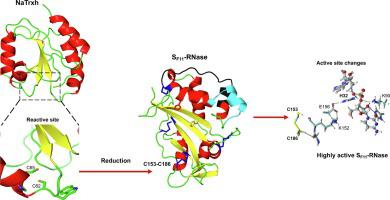
Thioredoxins are regulatory proteins that reduce disulfide bonds on target proteins. NaTrxh, which belongs to the plant thioredoxin family h subgroup 2, interacts and reduces the S-RNase enhancing its ribonuclease activity seven-fold, resulting an essential protein for pollen rejection in Nicotiana. Here, the crystal structure of NaTrxh at 1.7 Å by X-ray diffraction is reported. NaTrxh conserves the typical fold observed in other thioredoxins from prokaryotes and eukaryotes, but it contains extensions towards both N- and C-termini. The NaTrxh N-terminal extension participates in the reduction of S-RNase, and in the structure reported here, this is orientated towards the reactive site. The interaction between SF11-RNase and the NaTrxh N-terminal was simulated and the short-lived complex observed lasted for a tenth of ns. Moreover, we identified certain amino acids as SF11-RNase-E155 and NaTrxh-M104 as good candidates to contribute to the stability of the complex. Furthermore, we simulated the reduction of the C153-C186 SF11-RNase disulfide bond and observed subtle changes that affect the entire core, which might explain the increase in the ribonuclease activity of S-RNase when it is reduced by NaTrxh.
中文翻译:

来自 Nicotiana alata 的 NaTrxh 的高分辨率晶体结构及其与 S-RNase 的相互作用。
硫氧还蛋白是调节蛋白,可减少靶蛋白上的二硫键。NaTrxh 属于植物硫氧还蛋白家族h亚组 2,与 S-RNase 相互作用并降低其核糖核酸酶活性七倍,从而导致 烟草花粉排斥的必需蛋白质。 在这里,报告了通过 X 射线衍射在 1.7 Å 处 NaTrxh 的晶体结构。NaTrxh 保留了在原核生物和真核生物的其他硫氧还蛋白中观察到的典型折叠,但它包含向 N 和 C 末端的延伸。NaTrxh N 端延伸参与 S-RNase 的减少,并且在此处报告的结构中,它面向反应位点。S F11之间的互动-RNase 和 NaTrxh N 端被模拟,观察到的短寿命复合物持续了十分之一纳秒。此外,我们将某些氨基酸确定为 S F11 -RNase-E155 和 NaTrxh-M104 作为有助于复合物稳定性的良好候选者。此外,我们模拟了 C153-C186 S F11 -RNase 二硫键的还原,并观察到影响整个核心的细微变化,这可能解释了当 S-RNase 被 NaTrxh 还原时核糖核酸酶活性增加的原因。

























 京公网安备 11010802027423号
京公网安备 11010802027423号