当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective catalytic reduction of NOx with NH3 over TiO2 supported metal sulfate catalysts prepared via a sol–gel protocol
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-07-15 , DOI: 10.1039/d0nj02647f Yanke Yu 1, 2, 3, 4, 5 , Jiali Zhang 1, 2, 3, 4, 5 , Changwei Chen 1, 2, 3, 4, 5 , Mudi Ma 1, 2, 3, 4, 5 , Chi He 1, 2, 3, 4, 5 , Jifa Miao 5, 6, 7, 8, 9 , Huirong Li 5, 6, 7, 8, 9 , Jinsheng Chen 5, 6, 7, 8, 9
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-07-15 , DOI: 10.1039/d0nj02647f Yanke Yu 1, 2, 3, 4, 5 , Jiali Zhang 1, 2, 3, 4, 5 , Changwei Chen 1, 2, 3, 4, 5 , Mudi Ma 1, 2, 3, 4, 5 , Chi He 1, 2, 3, 4, 5 , Jifa Miao 5, 6, 7, 8, 9 , Huirong Li 5, 6, 7, 8, 9 , Jinsheng Chen 5, 6, 7, 8, 9
Affiliation
|
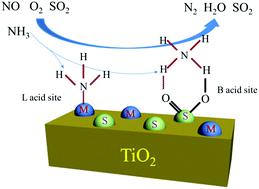
Due to the presence of SO2 in flue gas, SCR catalysts with high SO2 tolerance are desperately required. However, except vanadium-based catalysts, most metal oxide catalysts exhibit low SO2 tolerance. In this research study, CuSO4/TiO2, Fe2(SO4)3/TiO2, MnSO4/TiO2, Ce(SO4)2/TiO2 and CoSO4/TiO2 catalysts were prepared via a sol–gel protocol and used in the NH3-SCR reaction. Low-temperature N2-sorption, XRD, FT-IR, XPS, H2-TPR, NH3-TPD and in situ DRIFTS were used to characterize the physicochemical properties of the prepared catalysts. The presence of SO2 had little influence on the activity of all metal sulfate catalyst samples. CuSO4/TiO2 showed the highest SCR activity and apparent activation energy among the metal sulfate catalysts following the sequence of CuSO4/TiO2 < Fe2(SO4)3/TiO2 < MnSO4/TiO2 < Ce(SO4)2/TiO2 < CoSO4/TiO2. Characterization results indicated that the amount of acid sites was the main factor that influenced the catalytic activity of the metal sulfate catalysts. The NH3-SCR reaction of all metal sulfate catalysts followed the Eley–Rideal mechanism: NH3 was first adsorbed at the surface acid sites (Lewis and Brønsted acid sites), and then it reacted with gaseous NO and O2 to form N2 and H2O. The metal sulfate catalysts, especially CuSO4/TiO2, should be a promising candidate for vanadium-based SCR catalysts for NO reduction.
中文翻译:

通过溶胶-凝胶方案制备的TiO2负载的金属硫酸盐催化剂上的NH3选择性催化还原NOx
由于烟道气中存在SO 2,迫切需要具有高SO 2耐受性的SCR催化剂。但是,除了钒基催化剂外,大多数金属氧化物催化剂表现出低的SO 2耐受性。在本研究中,通过溶胶-法制备了CuSO 4 / TiO 2,Fe 2(SO 4)3 / TiO 2,MnSO 4 / TiO 2,Ce(SO 4)2 / TiO 2和CoSO 4 / TiO 2催化剂。凝胶协议,用于NH 3-SCR反应。利用低温N 2吸附,XRD,FT-IR,XPS,H 2 -TPR,NH 3 -TPD和原位DRIFTS表征了所制备催化剂的理化性质。SO 2的存在对所有金属硫酸盐催化剂样品的活性影响很小。的CuSO 4 /二氧化钛2表明在金属硫酸盐的催化剂中的最高SCR活性和表观活化能的CuSO的序列以下4 /二氧化钛2 <铁2(SO 4)3 /二氧化钛2 <的MnSO 4 /二氧化钛2<Ce(SO 4)2 / TiO 2 <CoSO 4 / TiO 2。表征结果表明,酸性部位的数量是影响金属硫酸盐催化剂催化活性的主要因素。所有金属硫酸盐催化剂的NH 3 -SCR反应遵循Eley-Rideal机理:NH 3首先被吸附在表面酸性位点(Lewis和Brønsted酸性位点),然后与气态NO和O 2反应形成N 2。和H 2 O的金属硫酸盐的催化剂,尤其是硫酸铜4 /二氧化钛2,应该是钒基SCR催化剂用于NO还原的有希望的候选者。
更新日期:2020-08-17
中文翻译:

通过溶胶-凝胶方案制备的TiO2负载的金属硫酸盐催化剂上的NH3选择性催化还原NOx
由于烟道气中存在SO 2,迫切需要具有高SO 2耐受性的SCR催化剂。但是,除了钒基催化剂外,大多数金属氧化物催化剂表现出低的SO 2耐受性。在本研究中,通过溶胶-法制备了CuSO 4 / TiO 2,Fe 2(SO 4)3 / TiO 2,MnSO 4 / TiO 2,Ce(SO 4)2 / TiO 2和CoSO 4 / TiO 2催化剂。凝胶协议,用于NH 3-SCR反应。利用低温N 2吸附,XRD,FT-IR,XPS,H 2 -TPR,NH 3 -TPD和原位DRIFTS表征了所制备催化剂的理化性质。SO 2的存在对所有金属硫酸盐催化剂样品的活性影响很小。的CuSO 4 /二氧化钛2表明在金属硫酸盐的催化剂中的最高SCR活性和表观活化能的CuSO的序列以下4 /二氧化钛2 <铁2(SO 4)3 /二氧化钛2 <的MnSO 4 /二氧化钛2<Ce(SO 4)2 / TiO 2 <CoSO 4 / TiO 2。表征结果表明,酸性部位的数量是影响金属硫酸盐催化剂催化活性的主要因素。所有金属硫酸盐催化剂的NH 3 -SCR反应遵循Eley-Rideal机理:NH 3首先被吸附在表面酸性位点(Lewis和Brønsted酸性位点),然后与气态NO和O 2反应形成N 2。和H 2 O的金属硫酸盐的催化剂,尤其是硫酸铜4 /二氧化钛2,应该是钒基SCR催化剂用于NO还原的有希望的候选者。


























 京公网安备 11010802027423号
京公网安备 11010802027423号