当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct catalytic asymmetric synthesis of α-chiral primary amines.
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-07-15 , DOI: 10.1039/c9cs00921c Qin Yin 1 , Yongjie Shi , Jingxin Wang , Xumu Zhang
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-07-15 , DOI: 10.1039/c9cs00921c Qin Yin 1 , Yongjie Shi , Jingxin Wang , Xumu Zhang
Affiliation
|
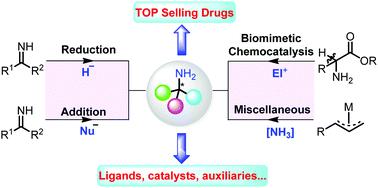
α-Chiral primary amines are one among the most valuable and versatile building blocks for the synthesis of numerous amine-containing pharmaceuticals and natural compounds. They also serve as chiral ligands or organo-catalysts for asymmetric catalysis. However, most of the existing chemocatalytic methods toward enantiopure primary amines rely on multistep manipulations on N-substituted substrates, which are not ideally atom-economical and cost-effective. Among the catalytic methods including the asymmetric transformations of the pre-prepared or in situ formed NH imines, biomimetic chemocatalysis inspired by enzymatic transaminations has recently emerged as an appealing and straightforward method to access chiral primary amines. This tutorial review highlights the state-of-the-art catalytic methods for the direct asymmetric synthesis of α-chiral primary amines and demonstrates their utility in the construction of molecular complexities, which may attract extensive attention and inspire applications in synthetic and medicinal chemistry.
中文翻译:

α-手性伯胺的直接催化不对称合成。
α-手性伯胺是合成许多含胺药物和天然化合物的最有价值和用途最广泛的组成部分之一。它们还用作不对称催化的手性配体或有机催化剂。然而,大多数现有的对映纯伯胺的化学催化方法大多数都依赖于对N-取代的底物的多步操作,这在理想情况下不是原子经济的和成本有效的。在催化方法中,包括预先制备或原位的不对称转化由于形成了NH亚胺,由酶促氨基转移引发的仿生化学催化最近成为吸引手性伯胺的一种吸引人且直接的方法。本教程概述重点介绍了用于α-手性伯胺直接不对称合成的最新催化方法,并展示了它们在构建分子复杂性中的实用性,这可能引起广泛关注并激发在合成和药物化学中的应用。
更新日期:2020-09-01
中文翻译:

α-手性伯胺的直接催化不对称合成。
α-手性伯胺是合成许多含胺药物和天然化合物的最有价值和用途最广泛的组成部分之一。它们还用作不对称催化的手性配体或有机催化剂。然而,大多数现有的对映纯伯胺的化学催化方法大多数都依赖于对N-取代的底物的多步操作,这在理想情况下不是原子经济的和成本有效的。在催化方法中,包括预先制备或原位的不对称转化由于形成了NH亚胺,由酶促氨基转移引发的仿生化学催化最近成为吸引手性伯胺的一种吸引人且直接的方法。本教程概述重点介绍了用于α-手性伯胺直接不对称合成的最新催化方法,并展示了它们在构建分子复杂性中的实用性,这可能引起广泛关注并激发在合成和药物化学中的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号