Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-07-15 , DOI: 10.1016/j.jorganchem.2020.121414 Yongxia Tan , Ning Li , Shuxuan Kong , Xuezhong Zhang , Shuhao Zhang , Zhijie Zhang , Zemin Xie
|
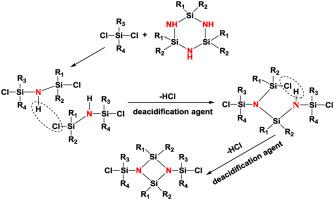
A novel convenient synthesis process for 1,3-bis(chlorodiorganosilyl)-cyclodisilazanes is developed via an intermolecular dehydrochlorination of 1,3-dichloro-tetraorgano-disilazane, in the presence of a strong organic alkaline deacidification agent 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). This procedure involves a one-step process under mild synthesis condition, with higher production efficiency and product purity as compared to those of the previously reported methods. The formation of 1,3-bis(chlorodiorganosilyl)-tetraorgano-cyclodisilazane occurs via the primary dehydrohalogenation of intermolecular 1,3-dichloro-tetraorgano-disilazanes to trisilylamine structure with a subsequent ring closure. The silicon atoms with different exocyclic or endocyclic substituents are closely related to the steric hindrance of the substituents. Dehydrochlorination reactions occur more readily on the chlorine atoms attached to the silicon atoms with substituents possessing relatively low steric hindrance. Four 1,3-dichloro-tetraorgano-disilazanes for the synthesis of 1,3-bis(chlorodiorganosilyl)-cyclodisilazanes are prepared. The investigation into the reaction mechanism shows that the equilibrium reaction of cyclosilazane with diorgano-dichlorosilane is a more straightforward and efficient method in the preparation of 1,3-dichloro-tetraorgano-disilazanes, as compared to the trans-silylation reaction of hexamethyl-disilazane with diorgano-dichlorosilane.
中文翻译:

的1,3-双合成(chlorodiorganosilyl)-cyclodisilazane通过在脱酸剂的存在下1,3-二氯四有机-二硅氮烷的脱氢氯化反应
在强有机碱性脱酸剂1,8-二氮杂双环[5.4]的存在下,通过1,3-二氯-四有机-二硅氮烷的分子间脱氯化氢,开发了一种新型的1,3-双(氯二有机甲硅烷基)-环二硅氮烷的便捷合成方法。.0] undec-7-ene(DBU)。该步骤涉及在温和的合成条件下的一步法,与先前报道的方法相比,具有更高的生产效率和产物纯度。1,3-双(氯二有机甲硅烷基)-四有机-环二硅氮烷的形成通过分子间的1,3-二氯-四有机-二硅氮烷的初步脱氢卤化为三甲硅烷基胺结构,并随后进行闭环。具有不同的环外或环内取代基的硅原子与取代基的空间位阻密切相关。在具有相对低位阻的取代基连接到硅原子的氯原子上更容易发生脱氯化氢反应。制备用于合成1,3-双(氯二有机甲硅烷基)-环二硅氮烷的四个1,3-二氯-四有机二硅氮烷。对反应机理的研究表明,与反式相比,环硅氮烷与二有机二氯硅烷的平衡反应是制备1,3-二氯四有机二硅氮烷的更直接有效的方法。六甲基二硅氮烷与二有机二氯硅烷的硅烷化反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号