Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-07-15 , DOI: 10.1016/j.jorganchem.2020.121417 Liliana Damas , Fábio M.S. Rodrigues , Andreia C.S. Gonzalez , Rui M.B. Carrilho , Marta Pineiro , Mariette M. Pereira
|
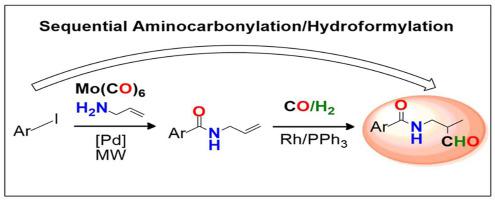
The sustainable synthesis of highly functionalised formylcarboxamide compounds with biological relevance is reported through a sequential aminocarbonylation/hydroformylation approach. The optimisation of palladium-catalysed aminocarbonylation of iodoaromatic substrates, using allylamine as nucleophile was first performed, with molybdenum hexacarbonyl as alternative CO source versus gaseous carbon monoxide. The combination of microwave irradiation with molybdenum hexacarbonyl allowed to selectively prepare a set of N-heterocyclic-based allylcarboxamides. Subsequent rhodium-catalysed hydroformylation of the allylcarboxamide intermediates led to the preparation of new pyridine, pyrazoline and chalcone derivatives containing both carboxamide and formyl moieties.
中文翻译:

连续催化羰基化反应可可持续合成生物相关实体
通过连续的氨基羰基化/加氢甲酰化方法报道了具有生物学相关性的高功能化甲酰甲酰胺化合物的可持续合成。首先使用烯丙基胺作为亲核试剂,以六羰基钼作为替代CO源(相对于气态一氧化碳),进行了钯催化的碘代芳族底物的氨基羰基化的优化。微波辐射与六羰基钼的组合允许选择性地制备一组基于N-杂环的烯丙基甲酰胺。烯丙基羧酰胺中间体的随后铑催化的加氢甲酰化反应导致制备了同时含有羧酰胺和甲酰基部分的新吡啶,吡唑啉和查尔酮衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号