当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Manipulating the regioselectivity of a Solanum lycopersicum epoxide hydrolase for the enantioconvergent synthesis of enantiopure alkane- and alkene-1,2-diols
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-07-14 , DOI: 10.1039/d0cy00990c Bochun Hu 1, 2, 3, 4, 5 , Die Hu 4, 5, 6, 7 , Dong Zhang 1, 2, 3, 4, 5 , Zheng Wen 1, 2, 3, 4, 5 , Jia Zang 7, 8, 9 , Minchen Wu 4, 5, 6, 7
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-07-14 , DOI: 10.1039/d0cy00990c Bochun Hu 1, 2, 3, 4, 5 , Die Hu 4, 5, 6, 7 , Dong Zhang 1, 2, 3, 4, 5 , Zheng Wen 1, 2, 3, 4, 5 , Jia Zang 7, 8, 9 , Minchen Wu 4, 5, 6, 7
Affiliation
|
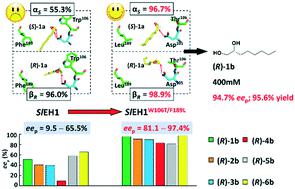
The hydrolysis of epoxides by epoxide hydrolases (EHs) is a sustainable approach to synthesize chiral vicinal diols. However, few EHs hitherto reported can catalyse the enantioconvergent synthesis of enantiopure alkane- and alkene-1,2-diols, due to the strict desire for regiocomplementarity. To actualize the enantioconvergent hydrolysis of rac-1,2-epoxyoctane (1a), the regioselectivity (αS, 55.3%) of SlEH1 for (S)-1a was manipulated via site-directed mutagenesis. Ten specific residues lining the substrate-binding pocket of SlEH1 were identified based on the computer-aided design, each of which was replaced by seven residues, respectively. Among 66 single-site mutants, SlEH1W106L, SlEH1W106T, SlEH1F109I, SlEH1M180V, SlEH1F189I and SlEH1F189L were selected, by which rac-1a was hydrolysed into (R)-octane-1,2-diol (1b) with an eep range of 62.1–82.4%. After combinatorial mutagenesis and screening, one double-site mutant, SlEH1W106T/F189L, was obtained having the highest αS of 96.7%. The gram-scale enantioconvergent hydrolysis of 400 mM (51.3 g L−1) rac-1a was carried out using 200 mg mL−1 wet cells of E. coli/sleh1W106T/F189L at 20 °C for 24 h, producing (R)-1b with 94.7% eep and 95.6% yield. The substrate spectrum assay of SlEH1W106T/F189L towards 20 mM rac-1a–6a was conducted, producing (R)-1b–6b with 81.1–97.4% eep values. Furthermore, the molecular dynamics simulation analysis indicated that the regiopreference of SlEH1W106T/F189L attacking on the Cα of (S)-1a was greater than that of SlEH1, which was consistent with their αS values measured experimentally. This work engineered a superior double-site mutant, SlEH1W106T/F189L, for the enantioconvergent synthesis of a variety of chiral alkane- and alkene-1,2-diols, especially (R)-1b and 6b, with high eep values.
中文翻译:

操纵茄茄环氧化物水解酶的区域选择性,以对映体合成对映纯的烷烃-和烯烃-1,2-二醇
通过环氧化物水解酶(EH)水解环氧化物是合成手性邻位二醇的可持续方法。然而,由于对区域互补性的严格要求,迄今报道的很少有EHs可以催化对映纯的烷烃-和烯烃-1,2-二醇的对映收敛合成。为了具体化的水解对映外消旋-1,2-环氧辛烷(1A),区域选择性(α小号,55.3%)的S1中EH1为(小号) - 1A被操纵通过位点定向诱变。Sl的底物结合口袋内衬的十个特定残基EH1是根据计算机辅助设计确定的,每个分别被七个残基取代。在66个单点突变体中,选择了Sl EH1 W106L,Sl EH1 W106T,Sl EH1 F109I,Sl EH1 M180V,Sl EH1 F189I和Sl EH1 F189L,将rac - 1a水解为(R)-辛烷-1,2 -二醇(1b)的ee p范围为62.1–82.4%。经过组合诱变和筛选后,有一个双位点突变体,SL EH1 W106T / F189L,得到具有最高α小号的96.7%。使用200 mg mL -1的E. coli / sleh1 W106T / F189L湿细胞在20°C下进行400 mM(51.3 g L -1)rac - 1a的克级对映体水解24 h,产生(R)-1b,ee p为94.7%,产率为95.6%。进行了针对20 mM rac - 1a-6a的Sl EH1 W106T / F189L的底物光谱测定,产生(R)-1b–6b的ee p值为81.1–97.4%。此外,分子动力学模拟分析表明的regiopreference S1中EH1 W106T / F189L在C攻击α(的小号) - 1A比的更大的S1中EH1,这是与它们一致的α小号实验测量值。这项工作设计了一个优异的双位点突变体Sl EH1 W106T / F189L,用于对映体合成各种具有高ee值的手性链烷烃和链烯基1,2-二醇,尤其是(R)-1b和6bp值。
更新日期:2020-09-05
中文翻译:

操纵茄茄环氧化物水解酶的区域选择性,以对映体合成对映纯的烷烃-和烯烃-1,2-二醇
通过环氧化物水解酶(EH)水解环氧化物是合成手性邻位二醇的可持续方法。然而,由于对区域互补性的严格要求,迄今报道的很少有EHs可以催化对映纯的烷烃-和烯烃-1,2-二醇的对映收敛合成。为了具体化的水解对映外消旋-1,2-环氧辛烷(1A),区域选择性(α小号,55.3%)的S1中EH1为(小号) - 1A被操纵通过位点定向诱变。Sl的底物结合口袋内衬的十个特定残基EH1是根据计算机辅助设计确定的,每个分别被七个残基取代。在66个单点突变体中,选择了Sl EH1 W106L,Sl EH1 W106T,Sl EH1 F109I,Sl EH1 M180V,Sl EH1 F189I和Sl EH1 F189L,将rac - 1a水解为(R)-辛烷-1,2 -二醇(1b)的ee p范围为62.1–82.4%。经过组合诱变和筛选后,有一个双位点突变体,SL EH1 W106T / F189L,得到具有最高α小号的96.7%。使用200 mg mL -1的E. coli / sleh1 W106T / F189L湿细胞在20°C下进行400 mM(51.3 g L -1)rac - 1a的克级对映体水解24 h,产生(R)-1b,ee p为94.7%,产率为95.6%。进行了针对20 mM rac - 1a-6a的Sl EH1 W106T / F189L的底物光谱测定,产生(R)-1b–6b的ee p值为81.1–97.4%。此外,分子动力学模拟分析表明的regiopreference S1中EH1 W106T / F189L在C攻击α(的小号) - 1A比的更大的S1中EH1,这是与它们一致的α小号实验测量值。这项工作设计了一个优异的双位点突变体Sl EH1 W106T / F189L,用于对映体合成各种具有高ee值的手性链烷烃和链烯基1,2-二醇,尤其是(R)-1b和6bp值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号