Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
β-Carboline Derivatives Tackling Malaria: Biological Evaluation and Docking Analysis.
ACS Omega ( IF 4.1 ) Pub Date : 2020-07-13 , DOI: 10.1021/acsomega.0c01256 Varun Gorki 1 , Neha Sylvia Walter 1 , Rahul Singh 2 , Monika Chauhan 3 , Neelima Dhingra 3 , Deepak B Salunke 2 , Sukhbir Kaur 1
ACS Omega ( IF 4.1 ) Pub Date : 2020-07-13 , DOI: 10.1021/acsomega.0c01256 Varun Gorki 1 , Neha Sylvia Walter 1 , Rahul Singh 2 , Monika Chauhan 3 , Neelima Dhingra 3 , Deepak B Salunke 2 , Sukhbir Kaur 1
Affiliation
|
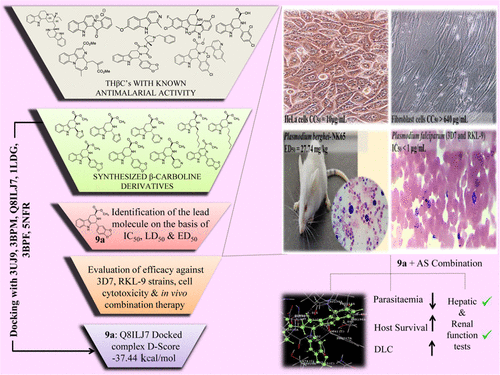
Increasing resistance to presently available antimalarial drugs urges the need to look for new promising compounds. The β-carboline moiety, present in several biologically active natural products and drugs, is an important scaffold for antimalarial drug discovery. The present study explores the antimalarial activity of a β-carboline derivative (1R,3S)-methyl 1-(benzo[d][1,3]dioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (9a) alone in vitro against Plasmodium falciparum and in vivo in combination therapy with the standard drug artesunate against Plasmodium berghei. Compound 9a inhibited both 3D7 and RKL-9 strains of P. falciparum with half-maximal inhibitory concentration (IC50) < 1 μg/mL, respectively. The compound was nontoxic (50% cytotoxic concentration (CC50) > 640 μg/mL) to normal dermal fibroblasts. Selectivity index was >10 against both the strains. The compound exhibited considerable in vivo antimalarial activity (median effective dose (ED50) = 27.74 mg/kg) in monotherapy. The combination of 9a (100 mg/kg) and artesunate (50 mg/kg) resulted in 99.69% chemosuppression on day 5 along with a mean survival time of 25.8 ± 4.91 days with complete parasite clearance. Biochemical studies indicated the safety of the HIT compound to hepatic and renal functions of mice. Molecular docking also highlighted the suitability of 9a as a potential antimalarial candidate.
中文翻译:

β-Carboline衍生物应对疟疾:生物学评估和对接分析。
对目前可用的抗疟药的抗药性增强,因此有必要寻找新的有前途的化合物。存在于几种具有生物活性的天然产物和药物中的β-咔啉部分是抗疟疾药物发现的重要支架。本研究探讨了β-咔啉衍生物(1 R,3 S)-甲基1-(苯并[ d ] [1,3]二氧戊-5-基)-2,3,4,9-四氢的抗疟活性-1 H-吡啶并[3,4- b ]吲哚-3-羧酸盐(9a)单独在体外对抗恶性疟原虫,在体内与标准药物青蒿琥酯联合治疗对抗伯氏疟原虫。化合物9a抑制恶性疟原虫的3D7和RKL-9菌株,其半数最大抑制浓度(IC 50)<1μg/ mL。该化合物对正常真皮成纤维细胞无毒(50%细胞毒性浓度(CC 50)> 640μg/ mL)。两种菌株的选择性指数均> 10。该化合物在单一疗法中表现出相当大的体内抗疟活性(中位有效剂量(ED 50)= 27.74 mg / kg)。9a的组合(100 mg / kg)和青蒿琥酯(50 mg / kg)在第5天产生了99.69%的化学抑制作用,平均生存时间为25.8±4.91天,具有完全的寄生虫清除率。生化研究表明,HIT化合物对小鼠肝和肾功能的安全性。分子对接也突出了9a作为潜在抗疟疾候选物的适用性。
更新日期:2020-07-28
中文翻译:

β-Carboline衍生物应对疟疾:生物学评估和对接分析。
对目前可用的抗疟药的抗药性增强,因此有必要寻找新的有前途的化合物。存在于几种具有生物活性的天然产物和药物中的β-咔啉部分是抗疟疾药物发现的重要支架。本研究探讨了β-咔啉衍生物(1 R,3 S)-甲基1-(苯并[ d ] [1,3]二氧戊-5-基)-2,3,4,9-四氢的抗疟活性-1 H-吡啶并[3,4- b ]吲哚-3-羧酸盐(9a)单独在体外对抗恶性疟原虫,在体内与标准药物青蒿琥酯联合治疗对抗伯氏疟原虫。化合物9a抑制恶性疟原虫的3D7和RKL-9菌株,其半数最大抑制浓度(IC 50)<1μg/ mL。该化合物对正常真皮成纤维细胞无毒(50%细胞毒性浓度(CC 50)> 640μg/ mL)。两种菌株的选择性指数均> 10。该化合物在单一疗法中表现出相当大的体内抗疟活性(中位有效剂量(ED 50)= 27.74 mg / kg)。9a的组合(100 mg / kg)和青蒿琥酯(50 mg / kg)在第5天产生了99.69%的化学抑制作用,平均生存时间为25.8±4.91天,具有完全的寄生虫清除率。生化研究表明,HIT化合物对小鼠肝和肾功能的安全性。分子对接也突出了9a作为潜在抗疟疾候选物的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号