当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thiazole‐tethered biaryls as fluorescent chemosensors for the selective detection of Fe3+ ions
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-14 , DOI: 10.1002/jhet.4093 Balasubramanian Mariammal 1 , Adaikalam Shylaja 1 , Sundaravel Vivek Kumar 1 , Stephen Raja Rubina 1 , Raju Ranjith Kumar 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-14 , DOI: 10.1002/jhet.4093 Balasubramanian Mariammal 1 , Adaikalam Shylaja 1 , Sundaravel Vivek Kumar 1 , Stephen Raja Rubina 1 , Raju Ranjith Kumar 1
Affiliation
|
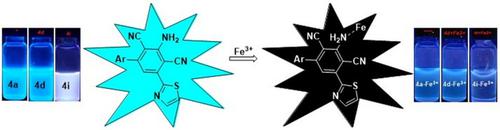
3‐Amino‐5‐(thiazol‐2‐yl)‐[1,1′‐biaryl]‐2,4‐dicarbonitriles have been synthesized employing a facile one‐pot pseudo four‐component domino strategy. All these thiazole‐tethered biaryls exhibited blue fluorescence under UV lamp. Based on the high relative quantum yield, three compounds namely, 4a, 4d, and 4i, were chosen to explore the metal interference studies. Against several metal ions, these three thiazole‐tethered biphenyl probes were found to be effective fluorescent chemosensors for the selective and sensitive detection of Fe3+ ions with a lower detection limit of 0.18, 0.12, and 0.16 μM, respectively.
中文翻译:

噻唑系联的联芳基作为荧光化学传感器,用于选择性检测Fe3 +离子
3-氨基5-(噻唑-2-基)-[1,1'-联芳基] -2,4-二碳腈采用一种简单的一锅法伪四组分多米诺策略合成。所有这些噻唑系联的联芳基在紫外灯下均显示蓝色荧光。基于较高的相对量子产率,选择了三种化合物4a,4d和4i进行金属干扰研究。对于三种金属离子,发现这三种噻唑系联苯探针是有效的荧光化学传感器,用于选择性和灵敏地检测Fe 3+离子,其检测下限分别为0.18、0.12和0.16μM。
更新日期:2020-07-14
中文翻译:

噻唑系联的联芳基作为荧光化学传感器,用于选择性检测Fe3 +离子
3-氨基5-(噻唑-2-基)-[1,1'-联芳基] -2,4-二碳腈采用一种简单的一锅法伪四组分多米诺策略合成。所有这些噻唑系联的联芳基在紫外灯下均显示蓝色荧光。基于较高的相对量子产率,选择了三种化合物4a,4d和4i进行金属干扰研究。对于三种金属离子,发现这三种噻唑系联苯探针是有效的荧光化学传感器,用于选择性和灵敏地检测Fe 3+离子,其检测下限分别为0.18、0.12和0.16μM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号