当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Haemoglobin(βK120C)–albumin trimer as an artificial O2 carrier with sufficient haemoglobin allostery
RSC Chemical Biology Pub Date : 2020-07-13 , DOI: 10.1039/d0cb00056f Yoshitsugu Morita 1 , Asuka Saito 1 , Jun Yamaguchi 1 , Teruyuki Komatsu 1
RSC Chemical Biology Pub Date : 2020-07-13 , DOI: 10.1039/d0cb00056f Yoshitsugu Morita 1 , Asuka Saito 1 , Jun Yamaguchi 1 , Teruyuki Komatsu 1
Affiliation
|
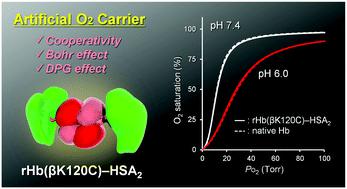
The allosteric O2 release of haemoglobin (Hb) allows for efficient O2 delivery from the lungs to the tissues. However, allostery is weakened in Hb-based O2 carriers because the chemical modifications of the Lys- and Cys-β93 residues prevent the quaternary transition of Hb. In this paper, we describe the synthesis and O2 binding properties of a recombinant Hb [rHb(βK120C)]–albumin heterotrimer that maintains sufficient Hb allostery. The rHb(βK120C) core, with two additional cysteine residues at the symmetrical positions on its protein surface, was expressed using yeast cells. The mutations did not influence either the O2 binding characteristics or the quaternary transition of Hb. Maleimide-activated human serum albumins (HSAs) were coupled with rHb(βK120C) at the two Cys-β120 positions, yielding the rHb(βK120C)–HSA2 trimer, in which the Cys-β93 residues were unreacted. Molecular dynamics simulation demonstrated that the HSA moiety does not interact with the amino acid residues around the haem pockets and the α1β2 surfaces of the rHb(βK120C) core, the alteration of which retards Hb allostery. Circular dichroism spectroscopy demonstrated that the quaternary transition between the relaxed (R) state and the tense (T) state of the Hb core occurred upon both the association and dissociation of O2. In phosphate-buffered saline solution (pH 7.4) at 37 °C, the rHb(βK120C)–HSA2 trimer exhibited a sigmoidal O2 equilibrium curve with the O2 affinity and cooperativity identical to those of native Hb (p50 = 12 Torr, n = 2.4). Moreover, we observed an equal Bohr effect and 2,3-diphosphoglycerate response in the rHb(βK120C)–HSA2 trimer compared with naked Hb.
中文翻译:

血红蛋白(βK120C)-白蛋白三聚体作为具有足够血红蛋白变构的人工O2载体
血红蛋白 (Hb)的变构 O 2释放允许将 O 2有效地从肺部输送到组织。然而,基于 Hb 的 O 2载体的变构被削弱,因为 Lys- 和 Cys-β93 残基的化学修饰阻止了 Hb 的四级转变。在本文中,我们描述了重组 Hb [rHb(βK120C)]-白蛋白异源三聚体的合成和 O 2结合特性,该异源三聚体保持足够的 Hb 变构。rHb(βK120C) 核心在其蛋白质表面的对称位置具有两个额外的半胱氨酸残基,使用酵母细胞表达。突变不影响 O 2结合特征或 Hb 的四级转变。马来酰亚胺活化的人血清白蛋白 (HSA) 在两个 Cys-β120 位置与 rHb(βK120C) 偶联,产生 rHb(βK120C)-HSA 2三聚体,其中 Cys-β93 残基未反应。分子动力学模拟表明,HSA 部分不与血红素袋周围的氨基酸残基和rHb(βK120C) 核心的 α 1 β 2表面相互作用,其改变会阻碍 Hb 变构。圆二色光谱表明,Hb 核心的松弛 (R) 态和紧张 (T) 态之间的四级跃迁发生在 O 2的缔合和解离时. 在 37 °C 的磷酸盐缓冲盐水溶液 (pH 7.4) 中,rHb(βK120C)–HSA 2三聚体表现出 S 形 O 2平衡曲线,其 O 2亲和力和协同性与天然 Hb的 O 2亲和力和协同性相同 ( p 50 = 12 Torr , n = 2.4)。此外,我们在 rHb(βK120C)-HSA 2三聚体中观察到与裸 Hb相同的玻尔效应和 2,3-二磷酸甘油酸反应。
更新日期:2020-08-06
中文翻译:

血红蛋白(βK120C)-白蛋白三聚体作为具有足够血红蛋白变构的人工O2载体
血红蛋白 (Hb)的变构 O 2释放允许将 O 2有效地从肺部输送到组织。然而,基于 Hb 的 O 2载体的变构被削弱,因为 Lys- 和 Cys-β93 残基的化学修饰阻止了 Hb 的四级转变。在本文中,我们描述了重组 Hb [rHb(βK120C)]-白蛋白异源三聚体的合成和 O 2结合特性,该异源三聚体保持足够的 Hb 变构。rHb(βK120C) 核心在其蛋白质表面的对称位置具有两个额外的半胱氨酸残基,使用酵母细胞表达。突变不影响 O 2结合特征或 Hb 的四级转变。马来酰亚胺活化的人血清白蛋白 (HSA) 在两个 Cys-β120 位置与 rHb(βK120C) 偶联,产生 rHb(βK120C)-HSA 2三聚体,其中 Cys-β93 残基未反应。分子动力学模拟表明,HSA 部分不与血红素袋周围的氨基酸残基和rHb(βK120C) 核心的 α 1 β 2表面相互作用,其改变会阻碍 Hb 变构。圆二色光谱表明,Hb 核心的松弛 (R) 态和紧张 (T) 态之间的四级跃迁发生在 O 2的缔合和解离时. 在 37 °C 的磷酸盐缓冲盐水溶液 (pH 7.4) 中,rHb(βK120C)–HSA 2三聚体表现出 S 形 O 2平衡曲线,其 O 2亲和力和协同性与天然 Hb的 O 2亲和力和协同性相同 ( p 50 = 12 Torr , n = 2.4)。此外,我们在 rHb(βK120C)-HSA 2三聚体中观察到与裸 Hb相同的玻尔效应和 2,3-二磷酸甘油酸反应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号