当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the mechanism of predominant urea formation from thermal degradation of CO2-loaded aqueous ethylenediamine.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0cp02178d Bohak Yoon 1 , Gyeong S Hwang
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0cp02178d Bohak Yoon 1 , Gyeong S Hwang
Affiliation
|
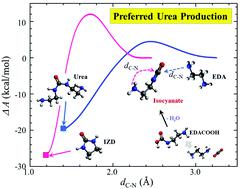
This study attempts to explain the well-known experimental observation that 1,3-bis(2-aminoethyl)urea (urea) is preferentially formed over the other major product, 2-imidazolidone (IZD), from thermal degradation of aqueous ethylenediamine (EDA) during the CO2 capture process. This is in direct contrast to the case of monoethanolamine (MEA), preferentially forming oxazolidinone (OZD), rather than urea, which undergoes further reactions leading to more stable products. Given their similar molecular structures, the different preferred degradation pathways of EDA and MEA impose an intriguing question regarding the underlying mechanism responsible for the distinct difference. Thermal degradation of both EDA and MEA tends to proceed mainly via formation of an isocyanate intermediate that may further undergo either cyclization to IZD (or OZD) or a reaction with EDA (or MEA) forming urea. For the EDA case, our first-principles simulations clearly demonstrate that the urea formation reaction is kinetically more, but thermodynamically less, favorable than the cyclization reaction; the opposite is true for the MEA case. Our further analysis shows that EDA–isocyanate is less prone to cyclization than MEA–isocyanate, which in turn allows for more facile urea formation. The reconfiguration dynamics of isocyanate is found to be governed by the dynamic nature of the interaction between its terminal group and surrounding solvent molecules. Our work highlights the importance of kinetic effects associated with the local structure and dynamics of solvent molecules around the intermediates that may significantly alter the degradation process of amine solvents.
中文翻译:

关于负载二氧化碳的乙二胺水溶液热降解形成主要尿素的机理。
这项研究试图解释众所周知的实验观察结果,即乙二胺水溶液(EDA)的热降解反应比其他主要产物2-咪唑啉酮(IZD)优先形成1,3-双(2-氨基乙基)脲(尿素) )在CO 2捕获过程中。这与单乙醇胺(MEA)的情况直接形成对比,后者优先形成恶唑烷酮(OZD)而不是尿素,后者会进一步反应生成更稳定的产物。考虑到它们相似的分子结构,EDA和MEA的不同优选降解途径对引起明显差异的潜在机制提出了一个有趣的问题。EDA和MEA的热降解主要通过以下途径进行形成异氰酸酯中间体,该中间体可能进一步环化成IZD(或OZD)或与EDA(或MEA)反应形成尿素。对于EDA情况,我们的第一原理模拟清楚地表明,与环化反应相比,尿素形成反应在动力学上更有利于热力学,而在热力学上却不那么有利。对于MEA情况则相反。我们的进一步分析表明,EDA-异氰酸酯比MEA-异氰酸酯更不容易环化,从而可以更轻松地形成尿素。发现异氰酸酯的重新构型动力学受其端基与周围溶剂分子之间相互作用的动力学性质支配。
更新日期:2020-08-05
中文翻译:

关于负载二氧化碳的乙二胺水溶液热降解形成主要尿素的机理。
这项研究试图解释众所周知的实验观察结果,即乙二胺水溶液(EDA)的热降解反应比其他主要产物2-咪唑啉酮(IZD)优先形成1,3-双(2-氨基乙基)脲(尿素) )在CO 2捕获过程中。这与单乙醇胺(MEA)的情况直接形成对比,后者优先形成恶唑烷酮(OZD)而不是尿素,后者会进一步反应生成更稳定的产物。考虑到它们相似的分子结构,EDA和MEA的不同优选降解途径对引起明显差异的潜在机制提出了一个有趣的问题。EDA和MEA的热降解主要通过以下途径进行形成异氰酸酯中间体,该中间体可能进一步环化成IZD(或OZD)或与EDA(或MEA)反应形成尿素。对于EDA情况,我们的第一原理模拟清楚地表明,与环化反应相比,尿素形成反应在动力学上更有利于热力学,而在热力学上却不那么有利。对于MEA情况则相反。我们的进一步分析表明,EDA-异氰酸酯比MEA-异氰酸酯更不容易环化,从而可以更轻松地形成尿素。发现异氰酸酯的重新构型动力学受其端基与周围溶剂分子之间相互作用的动力学性质支配。


























 京公网安备 11010802027423号
京公网安备 11010802027423号