当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of quinone imine and sulphur-containing compounds with antitumor and trypanocidal activities: redox and biological implications
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0md00072h Renata G Almeida 1 , Wagner O Valença 1, 2 , Luísa G Rosa 1 , Carlos A de Simone 3 , Solange L de Castro 4 , Juliana M C Barbosa 4 , Daniel P Pinheiro 5 , Carlos R K Paier 5 , Guilherme G C de Carvalho 5 , Claudia Pessoa 5 , Marilia O F Goulart 6 , Ammar Kharma 1, 7 , Eufrânio N da Silva Júnior 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0md00072h Renata G Almeida 1 , Wagner O Valença 1, 2 , Luísa G Rosa 1 , Carlos A de Simone 3 , Solange L de Castro 4 , Juliana M C Barbosa 4 , Daniel P Pinheiro 5 , Carlos R K Paier 5 , Guilherme G C de Carvalho 5 , Claudia Pessoa 5 , Marilia O F Goulart 6 , Ammar Kharma 1, 7 , Eufrânio N da Silva Júnior 1
Affiliation
|
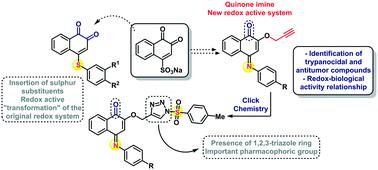
Ortho-Quinones represent a special class of redox active compounds associated with a spectrum of pronounced biological activities, including selective cytotoxicity and antimicrobial actions. The modification of the quinone ring by simple nitrogen and sulphur substitutions leads to several new classes of compounds with their own, distinct redox behaviour and equally distinct activities against cancer cell lines and Trypanosoma cruzi. Some of the compounds investigated show activity against T. cruzi at concentrations of 24.3 and 65.6 μM with a selectivity index of around 1. These results demonstrate that simple chemical modifications on the ortho-quinone ring system, in particular, by heteroatoms such as nitrogen and sulphur, transform these simple redox molecules into powerful cytotoxic agents with considerable “potential”, not only in synthesis and electrochemistry, but also, in a broader sense, in health sciences.
中文翻译:

具有抗肿瘤和杀锥虫活性的醌亚胺和含硫化合物的合成:氧化还原和生物学意义
邻醌代表一类特殊的氧化还原活性化合物,具有一系列显着的生物活性,包括选择性细胞毒性和抗菌作用。通过简单的氮和硫取代对醌环进行修饰,产生了几种新的化合物,它们具有自己独特的氧化还原行为以及同样独特的针对癌细胞系和克氏锥虫的活性。一些研究的化合物在浓度为 24.3 和 65.6 μM 时表现出对抗克氏锥虫的活性,选择性指数约为 1。这些结果表明,对邻醌环系统进行简单的化学修饰,特别是通过杂原子(例如氮和硫,将这些简单的氧化还原分子转化为强大的细胞毒性剂,不仅在合成和电化学方面,而且在更广泛的意义上,在健康科学方面具有相当大的“潜力”。
更新日期:2020-07-13
中文翻译:

具有抗肿瘤和杀锥虫活性的醌亚胺和含硫化合物的合成:氧化还原和生物学意义
邻醌代表一类特殊的氧化还原活性化合物,具有一系列显着的生物活性,包括选择性细胞毒性和抗菌作用。通过简单的氮和硫取代对醌环进行修饰,产生了几种新的化合物,它们具有自己独特的氧化还原行为以及同样独特的针对癌细胞系和克氏锥虫的活性。一些研究的化合物在浓度为 24.3 和 65.6 μM 时表现出对抗克氏锥虫的活性,选择性指数约为 1。这些结果表明,对邻醌环系统进行简单的化学修饰,特别是通过杂原子(例如氮和硫,将这些简单的氧化还原分子转化为强大的细胞毒性剂,不仅在合成和电化学方面,而且在更广泛的意义上,在健康科学方面具有相当大的“潜力”。



























 京公网安备 11010802027423号
京公网安备 11010802027423号