当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning regioselective oxidation toward phenol via atomically dispersed iron sites on carbon
Green Chemistry ( IF 9.8 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0gc01717e Yuxiao Ding 1, 2, 3, 4, 5 , Pengfei Zhang 4, 5, 6, 7 , Hailong Xiong 8, 9, 10, 11 , Xiaoyan Sun 1, 2, 3, 12, 13 , Alexander Klyushin 3, 14, 15 , Bingsen Zhang 11, 16, 17, 18 , Zigeng Liu 3, 19, 20, 21 , Jinshui Zhang 4, 5, 6, 7 , Huiyuan Zhu 4, 5, 6, 7 , Zhen-An Qiao 8, 9, 10, 11 , Saskia Heumann 1, 2, 3 , Sheng Dai 4, 5, 6, 7
Green Chemistry ( IF 9.8 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0gc01717e Yuxiao Ding 1, 2, 3, 4, 5 , Pengfei Zhang 4, 5, 6, 7 , Hailong Xiong 8, 9, 10, 11 , Xiaoyan Sun 1, 2, 3, 12, 13 , Alexander Klyushin 3, 14, 15 , Bingsen Zhang 11, 16, 17, 18 , Zigeng Liu 3, 19, 20, 21 , Jinshui Zhang 4, 5, 6, 7 , Huiyuan Zhu 4, 5, 6, 7 , Zhen-An Qiao 8, 9, 10, 11 , Saskia Heumann 1, 2, 3 , Sheng Dai 4, 5, 6, 7
Affiliation
|
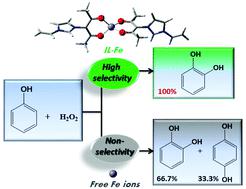
The development of environmentally benign catalysts for highly regioselective hydroxylation of phenol remains an unsolved challenge in both industry and academia because the electrophilic substitution of phenol simultaneously occurs on both ortho- and para-positions. Herein, we report a designed atomically dispersed iron-based heterogeneous catalyst, in which the iron species is coordinated by a functionalized ionic liquid monolayer on carbon nanotubes. The catalyst exhibits an unprecedented level of regioselectivity (>99%) towards the hydroxylation of phenol and displays a much better activity (TOF towards catechol productivity, 1.79 s−1) compared to the homogeneous free ion system (TOF towards catechol productivity, 0.44 s−1). Both experimental and theoretical investigations confirm that the catalytic oxidation with hydroperoxide undergoes a non-radical addition process and substitutes only the ortho-positions of phenol. This finding provides not only a quite active and selective catalyst for industrially very important reactions, but also a promising methodology of designing biomimetic iron-based heterogeneous catalysts at the atomic level.
中文翻译:

通过碳上原子分散的铁位点调节对苯酚的区域选择性氧化
由于苯酚的亲电子取代同时发生在对位和对位上,因此开发用于苯酚的高度区域选择性羟基化的环境友好型催化剂仍然是工业和学术界尚未解决的挑战。本文中,我们报道了一种设计原子分散的铁基非均相催化剂,其中铁物种通过碳纳米管上的功能化离子液体单层进行配位。与均相自由离子体系(TOF对邻苯二酚的生产率为0.44 s)相比,该催化剂对苯酚的羟基化具有前所未有的区域选择性(> 99%),并且具有更好的活性(对儿茶酚生产率的TOF为1.79 s -1)-1)。实验和理论研究均证实氢过氧化物催化氧化经历了非自由基加成过程,并且仅取代了苯酚的原位。这一发现不仅为工业上非常重要的反应提供了一种非常活性和选择性的催化剂,而且为在原子水平上设计仿生铁基非均相催化剂提供了一种有前途的方法。
更新日期:2020-09-21
中文翻译:

通过碳上原子分散的铁位点调节对苯酚的区域选择性氧化
由于苯酚的亲电子取代同时发生在对位和对位上,因此开发用于苯酚的高度区域选择性羟基化的环境友好型催化剂仍然是工业和学术界尚未解决的挑战。本文中,我们报道了一种设计原子分散的铁基非均相催化剂,其中铁物种通过碳纳米管上的功能化离子液体单层进行配位。与均相自由离子体系(TOF对邻苯二酚的生产率为0.44 s)相比,该催化剂对苯酚的羟基化具有前所未有的区域选择性(> 99%),并且具有更好的活性(对儿茶酚生产率的TOF为1.79 s -1)-1)。实验和理论研究均证实氢过氧化物催化氧化经历了非自由基加成过程,并且仅取代了苯酚的原位。这一发现不仅为工业上非常重要的反应提供了一种非常活性和选择性的催化剂,而且为在原子水平上设计仿生铁基非均相催化剂提供了一种有前途的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号