Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An internally eGFP-tagged α-adaptin is a fully functional and improved fiduciary marker for clathrin-coated pit dynamics.
Traffic ( IF 4.5 ) Pub Date : 2020-07-12 , DOI: 10.1111/tra.12755 Rosa E Mino 1 , Zhiming Chen 1 , Marcel Mettlen 1 , Sandra L Schmid 1
Traffic ( IF 4.5 ) Pub Date : 2020-07-12 , DOI: 10.1111/tra.12755 Rosa E Mino 1 , Zhiming Chen 1 , Marcel Mettlen 1 , Sandra L Schmid 1
Affiliation
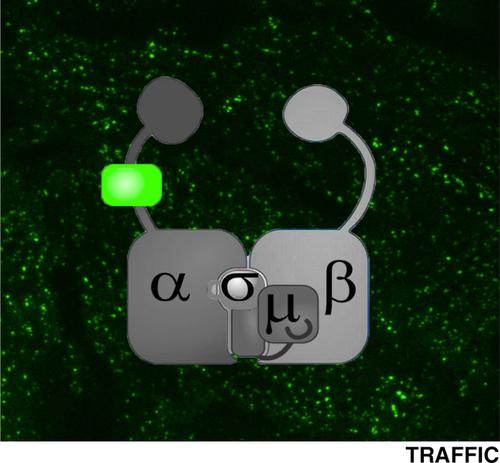
|
Clathrin mediated endocytosis (CME) has been extensively studied in living cells by quantitative total internal reflection fluorescence microscopy (TIRFM). Fluorescent protein fusions to subunits of the major coat proteins, clathrin light chains or the heterotetrameric adaptor protein (AP2) complexes, have been used as fiduciary markers of clathrin coated pits (CCPs). However, the functionality of these fusion proteins has not been rigorously compared. Here, we generated stable cells lines overexpressing mRuby‐CLCa and/or μ2‐eGFP, σ2‐eGFP, two markers currently in use, or a novel marker generated by inserting eGFP into the unstructured hinge region of the α subunit (α‐eGFP). Using biochemical and TIRFM‐based assays, we compared the functionality of the AP2 markers. All of the eGFP‐tagged subunits were efficiently incorporated into AP2 and displayed greater accuracy in image‐based CCP analyses than mRuby‐CLCa. However, overexpression of either μ2‐eGFP or σ2‐eGFP impaired transferrin receptor uptake. In addition, μ2‐eGFP reduced the rates of CCP initiation and σ2‐eGFP perturbed AP2 incorporation into CCPs and CCP maturation. In contrast, CME and CCP dynamics were unperturbed in cells overexpressing α‐eGFP. Moreover, α‐eGFP was a more sensitive and accurate marker of CCP dynamics than mRuby‐CLCa. Thus, our work establishes α‐eGFP as a robust, fully functional marker for CME.
中文翻译:

内部带有eGFP标记的α-adaptin是一种功能齐全的改进的基准标记,可用于网格蛋白包被的凹坑动力学。
网格蛋白介导的内吞作用(CME)已通过定量全内反射荧光显微镜(TIRFM)在活细胞中进行了广泛研究。荧光蛋白与主要外壳蛋白,网格蛋白轻链或异四聚体衔接蛋白(AP2)复合物的亚基融合,已被用作网格蛋白包被基坑(CCP)的基准标记。但是,尚未对这些融合蛋白的功能进行严格比较。在这里,我们生成了过表达mRuby‐CLCa和/或μ2‐eGFP,σ2‐eGFP,当前使用的两种标记或通过将eGFP插入α亚基的非结构化铰链区(α‐eGFP)产生的新标记的稳定细胞系。使用生化和基于TIRFM的分析,我们比较了AP2标记的功能。所有带有eGFP标签的亚基都有效地整合到了AP2中,在基于图像的CCP分析中显示出比mRuby‐CLCa更高的准确性。但是,μ2-eGFP或σ2-eGFP的过表达会损害转铁蛋白受体的摄取。此外,μ2-eGFP降低了CCP起始速率,而σ2-eGFP干扰了AP2掺入CCP和CCP成熟的过程。相反,在过表达α-eGFP的细胞中,CME和CCP动力学不受干扰。此外,与mRuby‐CLCa相比,α‐eGFP是CCP动态的更灵敏和准确的标记。因此,我们的工作将α-eGFP建立为CME的强大,功能齐全的标记。μ2-eGFP降低了CCP起始速率,而σ2-eGFP干扰了AP2加入CCP和CCP成熟的过程。相反,在过表达α-eGFP的细胞中,CME和CCP动力学不受干扰。此外,与mRuby‐CLCa相比,α‐eGFP是CCP动态的更灵敏和准确的标记。因此,我们的工作将α-eGFP建立为CME的强大,功能齐全的标记。μ2-eGFP降低了CCP起始速率,而σ2-eGFP干扰了AP2加入CCP和CCP成熟的过程。相反,在过表达α-eGFP的细胞中,CME和CCP动力学不受干扰。此外,与mRuby‐CLCa相比,α‐eGFP是CCP动态的更灵敏和准确的标记。因此,我们的工作将α-eGFP建立为CME的强大,功能齐全的标记。
更新日期:2020-08-19
中文翻译:

内部带有eGFP标记的α-adaptin是一种功能齐全的改进的基准标记,可用于网格蛋白包被的凹坑动力学。
网格蛋白介导的内吞作用(CME)已通过定量全内反射荧光显微镜(TIRFM)在活细胞中进行了广泛研究。荧光蛋白与主要外壳蛋白,网格蛋白轻链或异四聚体衔接蛋白(AP2)复合物的亚基融合,已被用作网格蛋白包被基坑(CCP)的基准标记。但是,尚未对这些融合蛋白的功能进行严格比较。在这里,我们生成了过表达mRuby‐CLCa和/或μ2‐eGFP,σ2‐eGFP,当前使用的两种标记或通过将eGFP插入α亚基的非结构化铰链区(α‐eGFP)产生的新标记的稳定细胞系。使用生化和基于TIRFM的分析,我们比较了AP2标记的功能。所有带有eGFP标签的亚基都有效地整合到了AP2中,在基于图像的CCP分析中显示出比mRuby‐CLCa更高的准确性。但是,μ2-eGFP或σ2-eGFP的过表达会损害转铁蛋白受体的摄取。此外,μ2-eGFP降低了CCP起始速率,而σ2-eGFP干扰了AP2掺入CCP和CCP成熟的过程。相反,在过表达α-eGFP的细胞中,CME和CCP动力学不受干扰。此外,与mRuby‐CLCa相比,α‐eGFP是CCP动态的更灵敏和准确的标记。因此,我们的工作将α-eGFP建立为CME的强大,功能齐全的标记。μ2-eGFP降低了CCP起始速率,而σ2-eGFP干扰了AP2加入CCP和CCP成熟的过程。相反,在过表达α-eGFP的细胞中,CME和CCP动力学不受干扰。此外,与mRuby‐CLCa相比,α‐eGFP是CCP动态的更灵敏和准确的标记。因此,我们的工作将α-eGFP建立为CME的强大,功能齐全的标记。μ2-eGFP降低了CCP起始速率,而σ2-eGFP干扰了AP2加入CCP和CCP成熟的过程。相反,在过表达α-eGFP的细胞中,CME和CCP动力学不受干扰。此外,与mRuby‐CLCa相比,α‐eGFP是CCP动态的更灵敏和准确的标记。因此,我们的工作将α-eGFP建立为CME的强大,功能齐全的标记。


























 京公网安备 11010802027423号
京公网安备 11010802027423号