Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Correlative cathodoluminescence electron microscopy bioimaging: towards single protein labelling with ultrastructural context.
Nanoscale ( IF 6.7 ) Pub Date : 2020-07-10 , DOI: 10.1039/d0nr02563a Kerda Keevend 1 , Toon Coenen , Inge K Herrmann
Nanoscale ( IF 6.7 ) Pub Date : 2020-07-10 , DOI: 10.1039/d0nr02563a Kerda Keevend 1 , Toon Coenen , Inge K Herrmann
Affiliation
|
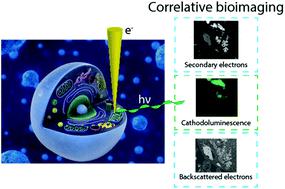
The understanding of living systems and their building blocks relies heavily on the assessment of structure–function relationships at the nanoscale. Ever since the development of the first optical microscope, the reliance of scientists across disciplines on microscopy has increased. The development of the first electron microscope and with it the access to information at the nanoscale has prompted numerous disruptive discoveries. While fluorescence imaging allows identification of specific entities based on the labelling with fluorophores, the unlabelled constituents of the samples remain invisible. In electron microscopy on the other hand, structures can be comprehensively visualized based on their distinct electron density and geometry. Although electron microscopy is a powerful tool, it does not implicitly provide information on the location and activity of specific organic molecules. While correlative light and electron microscopy techniques have attempted to unify the two modalities, the resolution mismatch between the two data sets poses major challenges. Recent developments in optical super resolution microscopy enable high resolution correlative light and electron microscopy, however, with considerable constraints due to sample preparation requirements. Labelling of specific structures directly for electron microscopy using small gold nanoparticles (i.e. immunogold) has been used extensively. However, identification of specific entities solely based on electron contrast, and the differentiation from endogenous dense granules, remains challenging. Recently, the use of correlative cathodoluminescence electron microscopy (CCLEM) imaging based on luminescent inorganic nanocrystals has been proposed. While nanometric resolution can be reached for both the electron and the optical signal, high energy electron beams are potentially damaging to the sample. In this review, we discuss the opportunities of (volumetric) multi-color single protein labelling based on correlative cathodoluminescence electron microscopy, and its prospective impact on biomedical research in general. We elaborate on the potential challenges of correlative cathodoluminescence electron microscopy-based bioimaging and benchmark CCLEM against alternative high-resolution correlative imaging techniques.
中文翻译:

相关的阴极发光电子显微镜生物成像:朝着具有超微结构背景的单个蛋白质标记。
对生命系统及其组成部分的理解在很大程度上取决于对纳米级结构与功能关系的评估。自从第一台光学显微镜的发展以来,科学家在各个学科上对显微镜的依赖就不断增加。第一台电子显微镜的发展以及随之而来的纳米级信息的获取已引发了众多破坏性发现。尽管荧光成像允许基于荧光团的标记识别特定实体,但样品的未标记成分仍然不可见。另一方面,在电子显微镜中,可以根据结构不同的电子密度和几何形状对结构进行全面可视化。尽管电子显微镜是一种强大的工具,它没有隐式提供有关特定有机分子的位置和活性的信息。尽管相关的光学和电子显微镜技术已试图统一这两种形式,但两个数据集之间的分辨率失配提出了重大挑战。光学超分辨率显微镜的最新发展使得高分辨率相关光和电子显微镜成为可能,但是由于样品制备要求而受到很大限制。直接使用小金纳米颗粒标记特定结构以用于电子显微镜(光学超分辨率显微镜的最新发展使得高分辨率相关光和电子显微镜成为可能,但是由于样品制备要求而受到很大限制。直接使用小金纳米颗粒标记特定结构以用于电子显微镜(光学超分辨率显微镜的最新发展使得高分辨率相关光和电子显微镜成为可能,但是由于样品制备要求而受到很大限制。直接使用小金纳米颗粒标记特定结构以用于电子显微镜(即免疫金)已被广泛使用。然而,仅基于电子对比度以及与内源致密颗粒的区别来鉴定特定实体仍然具有挑战性。近来,已经提出使用基于发光无机纳米晶体的相关阴极发光电子显微镜(CCLEM)成像。尽管电子和光信号都可以达到纳米级分辨率,但高能电子束可能会损坏样品。在这篇综述中,我们讨论了基于相关阴极发光电子显微镜的(体积)多色单蛋白标记的机会,以及它对生物医学研究的总体影响。
更新日期:2020-07-31
中文翻译:

相关的阴极发光电子显微镜生物成像:朝着具有超微结构背景的单个蛋白质标记。
对生命系统及其组成部分的理解在很大程度上取决于对纳米级结构与功能关系的评估。自从第一台光学显微镜的发展以来,科学家在各个学科上对显微镜的依赖就不断增加。第一台电子显微镜的发展以及随之而来的纳米级信息的获取已引发了众多破坏性发现。尽管荧光成像允许基于荧光团的标记识别特定实体,但样品的未标记成分仍然不可见。另一方面,在电子显微镜中,可以根据结构不同的电子密度和几何形状对结构进行全面可视化。尽管电子显微镜是一种强大的工具,它没有隐式提供有关特定有机分子的位置和活性的信息。尽管相关的光学和电子显微镜技术已试图统一这两种形式,但两个数据集之间的分辨率失配提出了重大挑战。光学超分辨率显微镜的最新发展使得高分辨率相关光和电子显微镜成为可能,但是由于样品制备要求而受到很大限制。直接使用小金纳米颗粒标记特定结构以用于电子显微镜(光学超分辨率显微镜的最新发展使得高分辨率相关光和电子显微镜成为可能,但是由于样品制备要求而受到很大限制。直接使用小金纳米颗粒标记特定结构以用于电子显微镜(光学超分辨率显微镜的最新发展使得高分辨率相关光和电子显微镜成为可能,但是由于样品制备要求而受到很大限制。直接使用小金纳米颗粒标记特定结构以用于电子显微镜(即免疫金)已被广泛使用。然而,仅基于电子对比度以及与内源致密颗粒的区别来鉴定特定实体仍然具有挑战性。近来,已经提出使用基于发光无机纳米晶体的相关阴极发光电子显微镜(CCLEM)成像。尽管电子和光信号都可以达到纳米级分辨率,但高能电子束可能会损坏样品。在这篇综述中,我们讨论了基于相关阴极发光电子显微镜的(体积)多色单蛋白标记的机会,以及它对生物医学研究的总体影响。

























 京公网安备 11010802027423号
京公网安备 11010802027423号