Current Analytical Chemistry ( IF 1.8 ) Pub Date : 2020-07-31 , DOI: 10.2174/1573407215666190131123029 Afnan E. Abdelrahman 1 , Hadir M. Maher 1 , Nourah Z. Alzoman 1
|
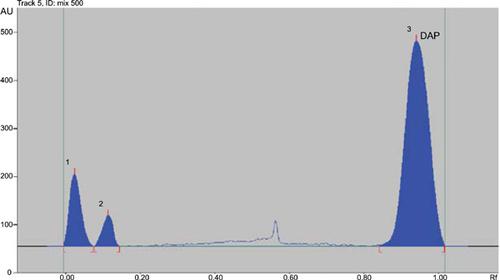
Background: Type 2 diabetes mellitus is an expanding health problem. Binary antidiabetic combinations of Metformin Hydrochloride (MET) with either Saxagliptin Hydrochloride (SAX), or Dapagliflozin (DAP) are widely used. Review of the literature revealed that no single HPTLC method has been reported for the simultaneous determination of MET, SAX, and DAP allowing the determination of binary mixtures of any two of the three cited drugs in their tablets using the same experimental conditions, an important advantage for quality control. The advantages of HPTLC method relies on the simultaneous analysis of a large number of samples in a shorter analysis time, less solvent consumption, and less expenses, compared with HPLC.
Objective: The objective of the proposed method is to develop and validate a single and simple HPTLC densitometric method for the simultaneous determination of MET, SAX, and DAP.
Methods: Separation was performed using aluminum HPTLC sheets coated with silica gel 60 F254 with a mobile phase consisting of a mixture of acetonitrile: 1% w/v ammonium acetate in methanol (9: 1, v/v). Scanning was performed at 210 nm.
Results and Discussion: Linearity of the method was assessed in the concentration range of 0.25-10 μg/band for SAX and DAP and 0.25-25 μg/band for MET. The method was fully validated as per the ICH guidelines. The proposed method provided error and deviation values of less than 2% assessing good accuracy and precision.
Conclusion: The method was successfully applied to the analysis of pharmaceutical tablets of MET/SAX, MET/DAP, and SAX/DAP with high specificity.
中文翻译:

HPTLC方法测定药品中的盐酸二甲双胍,盐酸沙格列汀和达格列净
背景:2型糖尿病是一个日益严重的健康问题。盐酸二甲双胍(MET)与盐酸沙格列汀(SAX)或Dapagliflozin(DAP)的二元抗糖尿病组合药物被广泛使用。文献综述表明,尚无单一的HPTLC方法可同时测定MET,SAX和DAP,从而可以在相同的实验条件下测定其片剂中三种被引药物中任意两种的二元混合物,这是一个重要的优势用于质量控制。与HPLC相比,HPTLC方法的优势在于可以在更短的分析时间,更少的溶剂消耗和更少的费用下同时分析大量样品。
目的:提出的方法的目的是开发和验证用于同时测定MET,SAX和DAP的单一且简单的HPTLC光密度法。
方法:使用涂有硅胶60 F254的HPTLC铝板进行分离,该板的流动相由乙腈:1%w / v乙酸铵的甲醇溶液(9:1,v / v)组成。扫描在210nm进行。
结果与讨论:该方法的线性在SAX和DAP浓度范围为0.25-10μg/ band和MET浓度范围为0.25-25μg/ band时进行了评估。该方法已按照ICH指南进行了充分验证。所提出的方法提供了小于2%的误差和偏差值,从而评估了良好的准确性和精密度。
结论:该方法已成功应用于高灵敏度的MET / SAX,MET / DAP和SAX / DAP药物片剂的分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号