当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Three Recently Diverging Duplicated Methyltransferases Exhibit Substrate-Dependent Regioselectivity Essential for Xantholipin Biosynthesis.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-07-10 , DOI: 10.1021/acschembio.0c00296 Lingxin Kong 1 , Qing Wang 1 , Weinan Yang 1 , Jufang Shen 1 , Yan Li 1 , Xiaoqing Zheng 1 , Lu Wang 2 , Yiwen Chu 2 , Zixin Deng 1 , Yit-Heng Chooi 3 , Delin You 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-07-10 , DOI: 10.1021/acschembio.0c00296 Lingxin Kong 1 , Qing Wang 1 , Weinan Yang 1 , Jufang Shen 1 , Yan Li 1 , Xiaoqing Zheng 1 , Lu Wang 2 , Yiwen Chu 2 , Zixin Deng 1 , Yit-Heng Chooi 3 , Delin You 1
Affiliation
|
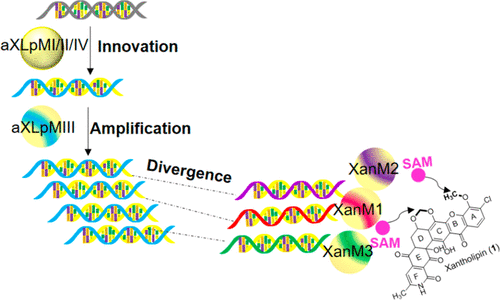
Polycyclic xanthones are characterized by highly oxygenated, angular hexacyclic frameworks and exhibit diverse biological activities. Although many of them have been isolated and chemically synthesized, the detailed biosynthetic machinery awaits discovery. Recently, xanthone construction in the xantholipin (1) pathway was shown to involve cryptic demethoxylation. This suggested a rationale for the existence of three O-methyltransferase (OMT) genes in the gene cluster, although there are only two O-methyl groups in the structure of 1. Here, in vivo and in vitro analysis have been used to show that the three paralogous OMTs, XanM1–M3, introduce individual methyl groups at specific points in the biosynthetic pathway. Each OMT can to some extent take over the role of the other OMTs, although they exhibit highly substrate-dependent regiospecificity. In addition, phylogenetic analysis suggests their evolution from a common ancestor. Four putative ancestral proteins were constructed, and one of them performed all the functions of XanM1–M3, while the others possessed more limited catalytic functions. The results suggest that a promiscuous common ancestor may have been able to catalyze all three reactions prior to gene duplication and functional divergence. The characterization of XanM1–M3 expands the enzyme inventory for polycyclic xanthone biosynthesis and suggests novel directed evolution approaches to diversifying natural product pathways.
中文翻译:

三个最近不同的重复甲基转移酶展示了黄嘌呤生物合成必不可少的底物依赖性区域选择性。
多环氧杂蒽的特征在于高度氧化的角六环骨架,并表现出多种生物活性。尽管其中许多已被分离并化学合成,但详细的生物合成机制仍有待发现。最近,黄嘌呤(1)途径中的黄酮构建被证明涉及隐秘的脱甲氧基化。这暗示了在基因簇中存在三个O-甲基转移酶(OMT)基因的基本原理,尽管在结构1中只有两个O-甲基。这里,在体内和体外分析表明,三个同源的OMT XanM1-M3在生物合成途径的特定位置引入了单独的甲基。每个OMT在某种程度上可以取代其他OMT,尽管它们表现出高度依赖底物的区域特异性。另外,系统发育分析表明它们是从共同祖先进化而来的。构建了四个推定的祖先蛋白,其中一个执行XanM1-M3的所有功能,而其他则具有更有限的催化功能。结果表明,在基因复制和功能分化之前,混杂的祖先可能已经能够催化所有三个反应。
更新日期:2020-08-21
中文翻译:

三个最近不同的重复甲基转移酶展示了黄嘌呤生物合成必不可少的底物依赖性区域选择性。
多环氧杂蒽的特征在于高度氧化的角六环骨架,并表现出多种生物活性。尽管其中许多已被分离并化学合成,但详细的生物合成机制仍有待发现。最近,黄嘌呤(1)途径中的黄酮构建被证明涉及隐秘的脱甲氧基化。这暗示了在基因簇中存在三个O-甲基转移酶(OMT)基因的基本原理,尽管在结构1中只有两个O-甲基。这里,在体内和体外分析表明,三个同源的OMT XanM1-M3在生物合成途径的特定位置引入了单独的甲基。每个OMT在某种程度上可以取代其他OMT,尽管它们表现出高度依赖底物的区域特异性。另外,系统发育分析表明它们是从共同祖先进化而来的。构建了四个推定的祖先蛋白,其中一个执行XanM1-M3的所有功能,而其他则具有更有限的催化功能。结果表明,在基因复制和功能分化之前,混杂的祖先可能已经能够催化所有三个反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号