当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Light-Mediated Chiral Phosphate Catalysis for Asymmetric Dicarbofunctionalization of Enamides
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-07-10 , DOI: 10.1021/acscatal.0c02660 Yang Shen 1 , Meng-Lan Shen 1 , Pu-Sheng Wang 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-07-10 , DOI: 10.1021/acscatal.0c02660 Yang Shen 1 , Meng-Lan Shen 1 , Pu-Sheng Wang 1
Affiliation
|
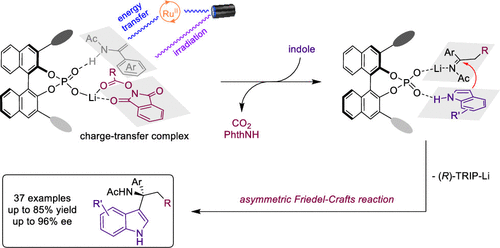
A light-mediated asymmetric dicarbofunctionalization of enamides with carboxylic-acid-derived redox-active esters (RAEs) and indoles has been established by using chiral lithium phosphate catalysis in the presence or absence of photoredox catalyst. This reaction features mild reaction conditions and broad substrate scopes, delivering a wide range of highly functionalized chiral amine derivatives. Mechanistic studies suggest that chiral lithium phosphate can serve as a pocket to accelerate the aggregation of enamide and RAE through hydrogen-bonding and coordination interaction, enabling the formation of a charge-transfer complex (CTC). Either enamide or CTC can be excited by direct irradiation or Ru(II)-mediated photosensitization to furnish chiral iminium intermediates for the asymmetric Friedel–Crafts reaction of indole.
中文翻译:

光介导的手性磷酸盐催化酰胺的不对称双碳官能化
在存在或不存在光氧化还原催化剂的情况下,通过使用手性磷酸锂催化,已经建立了酰胺与羧酸衍生的氧化还原活性酯(RAE)和吲哚的光介导的不对称双碳官能化。该反应具有温和的反应条件和广泛的底物范围,可提供多种高度官能化的手性胺衍生物。机理研究表明,手性磷酸锂可以作为口袋,通过氢键和配位相互作用加速烯酰胺和RAE的聚集,从而形成电荷转移复合物(CTC)。可以通过直接辐射或Ru(II)介导的光敏作用来激发烯酰胺或CTC,以为吲哚的不对称Friedel-Crafts反应提供手性亚胺中间体。
更新日期:2020-08-08
中文翻译:

光介导的手性磷酸盐催化酰胺的不对称双碳官能化
在存在或不存在光氧化还原催化剂的情况下,通过使用手性磷酸锂催化,已经建立了酰胺与羧酸衍生的氧化还原活性酯(RAE)和吲哚的光介导的不对称双碳官能化。该反应具有温和的反应条件和广泛的底物范围,可提供多种高度官能化的手性胺衍生物。机理研究表明,手性磷酸锂可以作为口袋,通过氢键和配位相互作用加速烯酰胺和RAE的聚集,从而形成电荷转移复合物(CTC)。可以通过直接辐射或Ru(II)介导的光敏作用来激发烯酰胺或CTC,以为吲哚的不对称Friedel-Crafts反应提供手性亚胺中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号