Immunology and Cell Biology ( IF 4 ) Pub Date : 2020-07-09 , DOI: 10.1111/imcb.12367 Jens Rettig 1 , Cosima T Baldari 2
|
Cytotoxic T lymphocytes (CTLs) are professional killers responsible for specific and efficient protection against viral pathogens and cancer. Restoring CTL function in these disorders, where infected or malignant cells have evolved strategies to evade killing, is the major goal of checkpoint inhibitor‐ or chimeric antigen receptor T‐cell‐based immunotherapy. The mediators of CTL‐mediated killing have been identified over 30 years ago as specialized secretory lysosomes known as lytic granules (LGs).1 The mechanisms that regulate LG biogenesis, release and action on target cells remain to be fully elucidated. A report recently published in Science by Bálint and colleagues,2 describing a new weapon in the cytotoxic arsenal of CTLs consisting of a lytic cargo wrapped in a glycoprotein shell, has brought an unexpected new level of complexity to the process of CTL‐mediated killing.
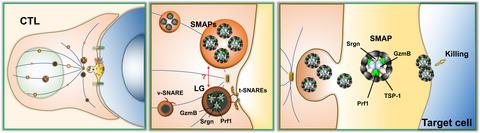
During their differentiation, CTLs are armed with an arsenal of toxic molecules that are delivered to target cells to activate their apoptotic demise. These molecules prominently include a group of potent serine proteases, the granzymes (Gzms), which are the principal mediators of the cytolytic activity of CTLs, and the pore‐forming protein perforin 1 (Prf1), which is responsible for Gzm delivery to the target cell. Gzms and Prf1 are packed onto a serglycin (Srgn) proteoglycan scaffold in LGs.3 Signaling by the T‐cell antigen receptor on CTL encounter of a cognate target cell results within seconds in the assembly of a specialized cell–cell interface, the immune synapse. Immune synapse formation is accompanied by the reorientation of the entire secretory apparatus, including LGs, toward the CTL‐target cell contact, concomitant with centrosome polarization.4 Upon arrival, LGs undergo fusion with the synaptic membrane through a process driven by the SNARE proteins syntaxin 11, SNAP‐23 and VAMP2/VAMP7, releasing their toxic contents into the synaptic cleft.5, 6 The subsequent uptake of Gzms by the target cell through pores generated by Prf1 initiates the apoptotic program that will eventually lead to its death.7 Although alternative models have been proposed for the final killing step, such as internalization of the Gzm–Prf–Srgn complexes in endosomes wherefrom Gzms are then released into the cytosol with the assistance of Prf1,8 LGs have been firmly established as the secretory organelle responsible for CTL‐mediated cytotoxicity. Bálint and colleagues challenge the current dogma with the identification of a novel cytotoxic particle, the supramolecular attack particle (SMAP), consisting of a core of Gzms, Prf1 and Srgn surrounded by a glycoprotein shell. These particles are preformed within acidic membrane compartments, are released by CTLs on contact with a cognate target and can autonomously elicit cell death in the absence of the CTL (Figure 1).2

Bálint et al . observed that human CTL clones engineered to express fluorescently tagged GzmB and labeled with wheat germ agglutinin, a lectin that does not recognize the carbohydrate moieties of GzmB, were able to transfer double‐positive puncta to antigen‐specific targets, indicating that GzmB participates in multiglycoprotein complexes, the SMAPs. Live imaging of CTLs plated on supported lipid bilayers coated with an anti‐CD3 antibody and ICAM‐1 (intercellular adhesion molecule 1) revealed that the SMAPs released onto the activating surface were stable for hours without fixation, supporting the notion that GzmB diffusion is prevented by its participation in SMAPs. Notably, GzmB+Prf1+ SMAPs remained attached to the supported lipid bilayers after CTL removal and had the ability to independently kill target cells.
Having established that, once released, the effectors of CTL cytotoxicity remain associated in stable particles with killing ability, the authors carried out a mass spectrometry analysis of SMAPs captured on supported lipid bilayers from T‐cell antigen receptor‐activated versus nonactivated CTLs. In addition to confirming the presence of GzmB and Prf1 in SMAPs from activated CTLs, the data revealed two interesting features: the absence of membrane‐associated proteins, including the lysosomal marker LAMP‐1 (lysosomal‐associated membrane protein 1) that decorates LGs, and the presence of thrombospondin‐1 (TSP‐1), a Ca2+‐binding glycoprotein of the extracellular matrix implicated in cell–cell and cell–matrix interactions.9 Imaging TSP‐1 in live CTLs showed that TSP‐1 colocalized with GzmB and Prf1 in intracellular acidic compartments and that it was released together with Prf1, suggesting that SMAPs are preassembled within the cell. Importantly, TSP‐1, of which only the C‐terminal 60‐kDa domain containing the Ca2+‐binding repeats was found to be associated with SMAPs, was essential for the assembly of SMAPs and their cytotoxic function. Using a combination of high‐resolution approaches to determine the structure of SMAPs, including direct stochastic optical reconstruction microscopy and cryo‐soft X‐ray tomography, Bálint and colleagues found that SMAPs are spherical entities of approximately 120 nm, tightly packed in intracellular multicore vesicles, consisting of a glycoprotein shell that includes TSP‐1 surrounding a core of GzmB, Prf1 and Srgn. Hence, SMAPs are a new class of cytotoxic particles that share with LGs their cytotoxic components but have a unique structure, a striking difference being the lack of a phospholipid membrane (Figure 1).
This exciting discovery not only provides a new paradigm of CTL‐mediated killing, but also raises a multitude of questions regarding the biogenesis of SMAPs and their specific role in CTL effector function that are expected to keep us busy over the next few years. First, how and where does the trafficking of TSP‐1, a protein destined for secretion to the extracellular matrix, intersect with the trafficking of Gzms, Prf1 and Srgn, which are routed to the specialized lysosomes that will eventually mature into LGs? One explanation could involve the deposition of extracellular matrix‐associated TSP‐1 on Gzm–Prf1–Srgn complexes when these are released from LGs upon fusion with the plasma membrane. However, the data clearly show an accumulation of TSP‐1 in acidic intracellular compartments that, based on their GzmB and Prf1 content, are likely to be LGs (or LG precursors). Thus, SMAPs are apparently assembled already prior to their release. One possible scenario is that the C‐terminal 60‐kDa domain of TSP‐1 represents a new isoform expressed specifically by CTLs. As such, it may provide a lysosome‐targeting signal, such as the one recognized by the cation‐independent mannose‐6‐phosphate receptor responsible for the lysosomal transport of Gzms and Prf1, that is not present in the canonical 145‐kDa isoform characterized in platelets. Alternatively, TSP‐1 that has been released at the cell surface might undergo internalization following binding to a surface receptor such as CD36 or CD47, with which it is known to interact,9 to undergo retrograde trafficking to lysosomes for subsequent SMAP assembly when these mature into LGs.
SMAPs have now been identified by Bálint et al . as unique to the well‐defined LGs, although, as both contain lytic capacity, it would suggest they share a mechanistic relationship in the CTL effector function. The multicore granules that contain SMAPs identified by cryo‐soft X‐ray tomography might represent a new type of LG, with the canonical dense‐core LGs releasing soluble Gzm–Prf1–Srgn complexes that are rapidly taken up by target cells through the Prf1 pathway, and the SMAP‐enriched "multicore LGs" releasing the same effectors but as insoluble multiglycoprotein complexes that remain stable and can slowly enter the target cell through an as yet unidentified mechanism. In support of this notion, the authors show that approximately equal amounts of GzmB and Prf1 are present in soluble versus SMAP‐associated forms in the supernatant of activated CTLs. The existence of two classes of cytotoxic particles, namely, the canonical LGs that rapidly release their content in a soluble form and the SMAPs, which upon release at the immune synapse maintain their lytic cargo stable for several hours, might considerably extend the efficacy of target cell killing by prolonging the time scale of the killing process even after the CTL has moved to another target.
How exactly cytolytic SMAPs are delivered to target cells and what directs their specificity remain to be investigated. TSP‐1 is known to bind several extracellular matrix components, including fibrinogen, fibronectin, laminin and collagens types V and VII. It also interacts with several integrins and the surface receptors CD36 and CD47.9 Other glycoprotein components of the shell, such as the β‐galactoside‐binding protein galectin‐1, could be implicated in SMAP uptake. In addition, to detect the SMAP shell Bálint and colleagues used wheat germ agglutinin, which binds to N‐acetylglucosamine, an amino sugar abundantly present in the extracellular matrix. Hence, SMAPs can potentially target a vast variety of cells independently of the antigen specificity of their CTL of origin. The fact that they are released into the synaptic cleft, which is tightly sealed through adhesive interactions stabilized by an F‐actin ring, suggests that they will largely target the cell with which the CTL has established a contact in an antigen‐specific fashion, thus ensuring the specificity of killing. However, CTLs are known to respond very rapidly to target cell recognition, releasing part of their LGs to readily move on to kill other cells.10 This implies that the long‐lived SMAPs may persist after CTL disengagement from its target, unless their uptake is very rapid, posing a potential threat to bystander healthy cells. Alternatively, CTLs releasing SMAPs might establish a longer contact with target cells in vivo , potentially explaining the heterogeneity of contact time observed in in vivo studies. These questions need to be answered to understand the specific function of SMAPs in the physiological context of CTL‐mediated killing.
An important implication of the release of stable cytotoxic particles by CTLs is the possibility to therapeutically exploit SMAPs to specifically kill cancer or virally infected cells without the need to manipulate CTLs. This poses two major challenges: to produce therapeutically relevant amounts of SMAPs and to engineer target‐cell specificity into the glycoprotein shell. Bálint and colleagues have shown that both CTLs and natural killer (NK) cells from peripheral blood release SMAPs. In contrast to CTLs, NK cell lines are available and could represent a viable source of SMAPs. Alternatively, elucidating the pathway of SMAP biogenesis will enable the engineering of cell lines that can be efficiently grown in a bioreactor to produce SMAPs. Manipulating the SMAP shell to render it target specific will first require mapping the molecular determinants essential for the integrity of the shell, in order to graft specificity in sites within TSP‐1 or other shell components that are not essential either to their folding or to their SMAP‐related function. There is a long and winding road ahead, but we may have the key to develop a sugar‐coated suicide pill for reluctant target cells. After all, a spoonful of sugar helps the medicine go down in the most delightful way .



























 京公网安备 11010802027423号
京公网安备 11010802027423号