Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic characterization of UDP‐glucuronic acid 4‐epimerase
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-07-09 , DOI: 10.1111/febs.15478 Annika J E Borg 1 , Alexander Dennig 1, 2 , Hansjörg Weber 3 , Bernd Nidetzky 1, 2
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-07-09 , DOI: 10.1111/febs.15478 Annika J E Borg 1 , Alexander Dennig 1, 2 , Hansjörg Weber 3 , Bernd Nidetzky 1, 2
Affiliation
|
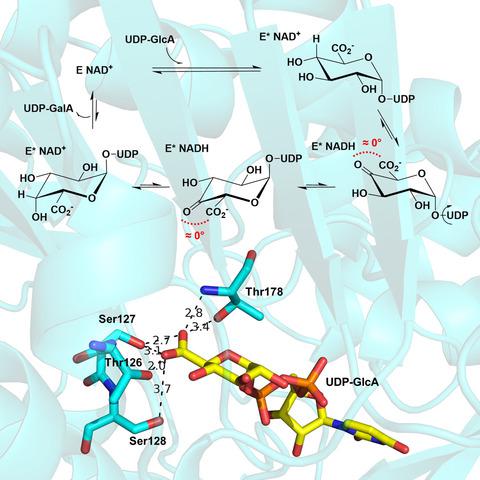
UDP‐glucuronic acid (UDP‐GlcA) is a central precursor in sugar nucleotide biosynthesis and common substrate for C4‐epimerases and decarboxylases releasing UDP‐galacturonic acid (UDP‐GalA) and UDP‐pentose products, respectively. Despite the different reactions catalyzed, the enzymes are believed to share mechanistic analogy rooted in their joint membership to the short‐chain dehydrogenase/reductase (SDR) protein superfamily: Oxidation at the substrate C4 by enzyme‐bound NAD+ initiates the catalytic pathway. Here, we present mechanistic characterization of the C4‐epimerization of UDP‐GlcA, which in comparison with the corresponding decarboxylation has been largely unexplored. The UDP‐GlcA 4‐epimerase from Bacillus cereus functions as a homodimer and contains one NAD+/subunit (kcat = 0.25 ± 0.01 s−1). The epimerization of UDP‐GlcA proceeds via hydride transfer from and to the substrate’s C4 while retaining the enzyme‐bound cofactor in its oxidized form (≥ 97%) at steady state and without trace of decarboxylation. The kcat for UDP‐GlcA conversion shows a kinetic isotope effect of 2.0 (±0.1) derived from substrate deuteration at C4. The proposed enzymatic mechanism involves a transient UDP‐4‐keto‐hexose‐uronic acid intermediate whose formation is rate‐limiting overall, and is governed by a conformational step before hydride abstraction from UDP‐GlcA. Precise positioning of the substrate in a kinetically slow binding step may be important for the epimerase to establish stereo‐electronic constraints in which decarboxylation of the labile β‐keto acid species is prevented effectively. Mutagenesis and pH studies implicate the conserved Tyr149 as the catalytic base for substrate oxidation and show its involvement in the substrate positioning step. Collectively, this study suggests that based on overall mechanistic analogy, stereo‐electronic control may be a distinguishing feature of catalysis by SDR‐type epimerases and decarboxylases active on UDP‐GlcA.
中文翻译:

UDP-葡萄糖醛酸4-表异构酶的机制表征
UDP-葡萄糖醛酸(UDP-GlcA)是糖核苷酸生物合成中的主要前体,是C4-表异构酶和脱羧酶的通用底物,分别释放UDP-半乳糖醛酸(UDP-GalA)和UDP-戊糖产品。尽管催化了不同的反应,但人们认为这些酶与短链脱氢酶/还原酶(SDR)蛋白超家族的共同成员有共同的机理:通过酶结合的NAD +在底物C4上的氧化启动了催化途径。在这里,我们介绍了UDP-GlcA的C4-表观异构化的机理表征,与相应的脱羧反应相比,在很大程度上尚待探索。蜡状芽孢杆菌的UDP-GlcA 4-表异构酶起同型二聚体的作用,并含有一个NAD +/亚基(k cat = 0.25±0.01 s -1)。UDP-GlcA的差向异构化是通过氢化物从底物的C4转移到底物的C4进行的,同时保持酶结合的辅因子处于稳定状态且处于氧化状态(≥97%),并且没有脱羧现象。该ķ猫UDP-GlcA转化的动力学同位素效应为2.0(±0.1),源于C4的底物氘。拟议的酶促机制涉及一个短暂的UDP-4-酮-己糖糖醛酸中间体,其形成总体上是限速的,并受从UDP-GlcA提取氢化物之前的构象步骤控制。底物在动力学上缓慢的结合步骤中的精确定位对于差向异构酶建立立体电子约束可能很重要,在该约束中有效防止不稳定的β-酮酸类物质的脱羧。诱变和pH研究表明,保守的Tyr149作为底物氧化的催化碱,表明其参与底物定位步骤。总的来说,这项研究表明,基于整体机制的类比,
更新日期:2020-07-09
中文翻译:

UDP-葡萄糖醛酸4-表异构酶的机制表征
UDP-葡萄糖醛酸(UDP-GlcA)是糖核苷酸生物合成中的主要前体,是C4-表异构酶和脱羧酶的通用底物,分别释放UDP-半乳糖醛酸(UDP-GalA)和UDP-戊糖产品。尽管催化了不同的反应,但人们认为这些酶与短链脱氢酶/还原酶(SDR)蛋白超家族的共同成员有共同的机理:通过酶结合的NAD +在底物C4上的氧化启动了催化途径。在这里,我们介绍了UDP-GlcA的C4-表观异构化的机理表征,与相应的脱羧反应相比,在很大程度上尚待探索。蜡状芽孢杆菌的UDP-GlcA 4-表异构酶起同型二聚体的作用,并含有一个NAD +/亚基(k cat = 0.25±0.01 s -1)。UDP-GlcA的差向异构化是通过氢化物从底物的C4转移到底物的C4进行的,同时保持酶结合的辅因子处于稳定状态且处于氧化状态(≥97%),并且没有脱羧现象。该ķ猫UDP-GlcA转化的动力学同位素效应为2.0(±0.1),源于C4的底物氘。拟议的酶促机制涉及一个短暂的UDP-4-酮-己糖糖醛酸中间体,其形成总体上是限速的,并受从UDP-GlcA提取氢化物之前的构象步骤控制。底物在动力学上缓慢的结合步骤中的精确定位对于差向异构酶建立立体电子约束可能很重要,在该约束中有效防止不稳定的β-酮酸类物质的脱羧。诱变和pH研究表明,保守的Tyr149作为底物氧化的催化碱,表明其参与底物定位步骤。总的来说,这项研究表明,基于整体机制的类比,



























 京公网安备 11010802027423号
京公网安备 11010802027423号