当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
sReactivation of CeO2‐based Catalysts in the HCl Oxidation Reaction: In situ Quantification of the Degree of Chlorination and Kinetic Modeling
ChemCatChem ( IF 4.5 ) Pub Date : 2020-07-10 , DOI: 10.1002/cctc.202000907 Yu Sun 1, 2 , Franziska Hess 3, 4 , Igor Djerdj 5 , Zheng Wang 1, 2 , Tim Weber 2, 6 , Yanglong Guo 1 , Bernd M. Smarsly 2, 6 , Herbert Over 2, 6
ChemCatChem ( IF 4.5 ) Pub Date : 2020-07-10 , DOI: 10.1002/cctc.202000907 Yu Sun 1, 2 , Franziska Hess 3, 4 , Igor Djerdj 5 , Zheng Wang 1, 2 , Tim Weber 2, 6 , Yanglong Guo 1 , Bernd M. Smarsly 2, 6 , Herbert Over 2, 6
Affiliation
|
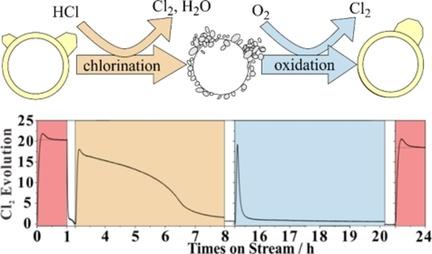
Deactivation of CeO2‐based catalysts in the HCl oxidation reaction proceeds via selective bulk chlorination of the active CeO2 component to form CeCl3×nH2O. We study the reactivation of two bulk‐chlorinated CeO2‐based Deacon catalysts by oxygen treatment at 430 °C, namely pure CeO2 and 20 mol % of CeO2 supported on preformed ZrO2 particles (20CeO2@ZrO2), with a dedicated experiment. In the flow reactor setup we determine in‐situ the degree of chlorination of the catalyst by quantifying down‐stream with in‐situ UV‐Vis spectroscopy the total amount of chlorine in the catalyst that is exchanged by reoxidation at 430 °C. The activity of deactivated 20CeO2@ZrO2 can be fully restored by oxygen exposure at 430 °C, while that of pure CeO2 declines steadily. Since the UV‐Vis analytics is fast and sensitive, we can follow the kinetics of reoxidation. To rationalize the observed kinetics, we develop a modified Johnson‐Mehl‐Avrami‐Kolmogorov (JMAK) model based on a nucleation‐and‐growth approach for the reoxidation of the catalyst starting from the chlorinated phase. The fast reoxidation kinetics of chlorinated 20CeO2@ZrO2 is traced to a fast nucleation rate.
中文翻译:

HCl氧化反应中基于CeO2的催化剂的再活化:氯化程度的原位定量和动力学建模
在HCl氧化反应中,基于CeO 2的催化剂的失活是通过活性CeO 2组分的选择性本体氯化而形成的CeCl 3 ×nH 2 O进行的。我们研究了通过氧气处理将两种基于本体的氯化CeO 2的Deacon催化剂进行的失活。通过专门的实验,在430°C下,即纯CeO 2和20 mol%的CeO 2负载在预先形成的ZrO 2颗粒(20CeO 2 @ZrO 2)上。在流动反应器设置中,我们确定原位通过使用原位UV-Vis光谱对下游进行量化来确定催化剂的氯化程度,即在430°C时通过再氧化交换的催化剂中的氯总量。失活的20CeO 2 @ZrO 2的活性可以通过在430°C下暴露于氧气来完全恢复,而纯CeO 2的活性则稳定下降。由于UV-Vis分析既快速又灵敏,我们可以跟踪再氧化的动力学。为了使观察到的动力学合理化,我们基于成核和生长方法开发了一种改良的Johnson-Mehl-Avrami-Kolmogorov(JMAK)模型,用于从氯化相开始的催化剂再氧化。氯化20CeO 2 @ZrO 2的快速再氧化动力学 可以追溯到快速成核速率。
更新日期:2020-07-10
中文翻译:

HCl氧化反应中基于CeO2的催化剂的再活化:氯化程度的原位定量和动力学建模
在HCl氧化反应中,基于CeO 2的催化剂的失活是通过活性CeO 2组分的选择性本体氯化而形成的CeCl 3 ×nH 2 O进行的。我们研究了通过氧气处理将两种基于本体的氯化CeO 2的Deacon催化剂进行的失活。通过专门的实验,在430°C下,即纯CeO 2和20 mol%的CeO 2负载在预先形成的ZrO 2颗粒(20CeO 2 @ZrO 2)上。在流动反应器设置中,我们确定原位通过使用原位UV-Vis光谱对下游进行量化来确定催化剂的氯化程度,即在430°C时通过再氧化交换的催化剂中的氯总量。失活的20CeO 2 @ZrO 2的活性可以通过在430°C下暴露于氧气来完全恢复,而纯CeO 2的活性则稳定下降。由于UV-Vis分析既快速又灵敏,我们可以跟踪再氧化的动力学。为了使观察到的动力学合理化,我们基于成核和生长方法开发了一种改良的Johnson-Mehl-Avrami-Kolmogorov(JMAK)模型,用于从氯化相开始的催化剂再氧化。氯化20CeO 2 @ZrO 2的快速再氧化动力学 可以追溯到快速成核速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号