Chem ( IF 23.5 ) Pub Date : 2020-07-10 , DOI: 10.1016/j.chempr.2020.06.021 Jing Wang , Cheng Qin , Jean-Philip Lumb , Xinjun Luan
|
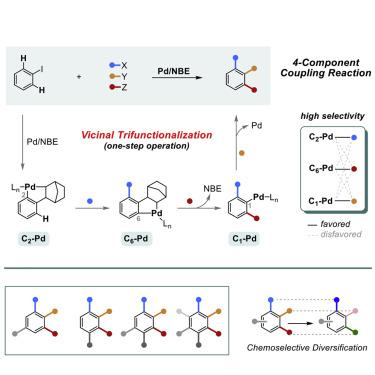
Polyfunctional arenes are an important part of the chemical value chain. To improve the efficiency of their synthesis, we have investigated a multicomponent approach built upon the Catellani platform. Here, we describe a 4-component coupling of aryl iodides lacking an ortho substituent that installs 3 discrete functional groups on the arene in a single step. The process is regio- and chemoselective and uncovers remote substituent effects that have a pronounced influence over intermediate Pd-(II) complexes. These intermediates have been a long-standing focus of the Catellani-reaction development, but persistent challenges have given rise to the well-known “ortho effect.” We now show that the ortho effect can be a positive element of reaction design, and that previously problematic iodides can now be used in complexity-generating transformations. In expanding the scope of the Catellani platform, we hope to provide mechanistic considerations to guide future reaction design, while also improving the environmental footprint of synthesizing polyfunctional arenes.
中文翻译:

通过4-组分卡泰拉尼反应区域选择性合成多官能团芳烃
多功能芳烃是化学价值链的重要组成部分。为了提高其合成效率,我们研究了基于Catellani平台的多组分方法。在这里,我们描述了一种缺少邻位取代基的芳基碘化物的4组分偶联,该取代基可在单个步骤中将3个离散的官能团安装在芳烃上。该过程具有区域选择性和化学选择性,并揭示了对中间Pd-(II)配合物具有明显影响的远程取代基效应。这些中间体一直是Catellani反应开发的长期重点,但是持续的挑战引起了众所周知的“邻位效应”。现在,我们证明邻位效果可能是反应设计的积极因素,以前有问题的碘化物现在可以用于产生复杂性的转化中。在扩大Catellani平台的范围时,我们希望提供机械方面的考虑,以指导未来的反应设计,同时也改善合成多功能芳烃的环境足迹。


























 京公网安备 11010802027423号
京公网安备 11010802027423号