Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2020-07-09 , DOI: 10.1016/j.apcatb.2020.119326 Weijie Zhu , Weixin Chen , Huanhuan Yu , Ye Zeng , Fangwang Ming , Hanfeng Liang , Zhoucheng Wang
|
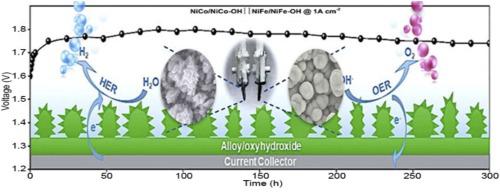
A big challenge in practical water splitting is the sluggish reaction kinetics at high current densities that essentially requires efficient electrocatalysts to lower the overpotentials. While exciting progress has been made in noble metal-based catalysts, earth-abundant materials that can actively catalyze the water splitting at high current densities (e.g. ≥500 mA cm−2) are rare. In this work, we show that a rational design of the catalysts could promote the charge transfer, facilitate the gas release, as well as boost the surface active sites, and therefore significantly enhance the electrocatalytic activity toward both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Using NiCo/NiCo−OH and NiFe/NiFe−OH as examples, we achieved ultralow overpotentials of 184 and 296 mV at 500 mA cm−2 in 1M KOH for HER and OER, respectively. More importantly, the alkaline electrolyzer based on these two materials is able to actively drive the overall water splitting at 1000 mA cm−2 for at least 300 h at a low cell voltage without significant performance decay, which is much superior to the state-of-the-art 20 % Pt/C||RuO2 combination. Our work points out a promising pathway to achieve inexpensive electrocatalysts for practical water splitting at high currents.
中文翻译:

NiCo / NiCo-OH和NiFe / NiFe-OH核壳纳米结构,用于大电流下的水分解电催化
实际水分解中的一大挑战是在高电流密度下反应动力学缓慢,这实质上需要有效的电催化剂来降低过电势。尽管在基于贵金属的催化剂方面取得了令人兴奋的进展,但是能够在高电流密度(例如≥500mA cm -2)下主动催化水分解的富含地球的材料却很少。在这项工作中,我们表明,合理设计催化剂可以促进电荷转移,促进气体释放,并提高表面活性位,因此显着增强了对析氢反应(HER)和氢的电催化活性。氧释放反应(OER)。以NiCo / NiCo-OH和NiFe / NiFe-OH为例,我们在500 mA cm处实现了184和296 mV的超低过电势HER和OER分别为1M KOH -2。更重要的是,基于这两种材料的碱性电解槽能够在低电池电压下主动驱动1000 mA cm -2的总水分解至少300 h,而不会出现明显的性能衰减,这远远优于电池的状态。最先进的20%Pt / C || RuO 2组合。我们的工作指出了一种有前途的途径,可以实现廉价的电催化剂,用于在高电流下进行实际的水分解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号