当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of the bis(cyclohexenone) core of (−)-lomaiviticin A
Chemical Science ( IF 8.4 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0sc02770g John A Rose 1 , Subham Mahapatra 1 , Xin Li 1 , Chao Wang 1 , Lei Chen 1 , Steven M Swick 1 , Seth B Herzon 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0sc02770g John A Rose 1 , Subham Mahapatra 1 , Xin Li 1 , Chao Wang 1 , Lei Chen 1 , Steven M Swick 1 , Seth B Herzon 1, 2
Affiliation
|
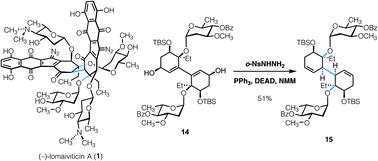
(−)-Lomaiviticin A is a complex C2-symmetric bacterial metabolite comprising two diazotetrahydrobenzo[b]fluorene (diazofluorene) residues and four 2,6-dideoxy glycosides, α-L-oleandrose and N,N-dimethyl-β-L-pyrrolosamine. The two halves of lomaiviticin A are linked by a single carbon–carbon bond oriented syn with respect to the oleandrose residues. While many advances toward the synthesis of lomaiviticin A have been reported, including synthesis of the aglycon, a route to the bis(cyclohexenone) core bearing any of the carbohydrate residues has not been disclosed. Here we describe a short route to a core structure of lomaiviticin A bearing two α-L-oleandrose residues. The synthetic route features a Stille coupling to form the conjoining carbon–carbon bond of the target and a double reductive transposition to establish the correct stereochemistry at this bond. Two synthetic routes were developed to elaborate the reductive transposition product to the bis(cyclohexenone) target. The more efficient pathway features an interrupted Barton vinyl iodide synthesis followed by oxidative elimination of iodide to efficiently establish the enone functionalities in the target. The bis(cyclohexenone) product may find use in a synthesis of lomaiviticin A itself.
中文翻译:

(-)-lomaiviticin A的双(环己烯酮)核心的合成
( - ) - Lomaiviticin A是一个复杂的Ç 2 -对称的细菌代谢物,其包括两个diazotetrahydrobenzo [ b ]芴(diazofluorene)残基和四个-2,6-二脱氧苷,α-大号-oleandrose和Ñ,ñ -二甲基- β-大号-吡咯烷胺。lomaiviticin A的两半通过单个碳-碳键定向的syn连接关于夹竹桃糖残基。尽管已经报道了合成lomaiviticin A的许多进展,包括糖苷配基的合成,但是尚未公开通往带有任何碳水化合物残基的双(环己烯酮)核心的途径。在这里,我们描述了一条带有两个α- L的lomaiviticin A核心结构的捷径-oleandrose残基。合成途径具有斯蒂勒偶联作用,形成靶标的碳-碳键,以及双重还原性转座,以在该键处建立正确的立体化学。开发了两种合成途径以阐明还原性转座产物对双(环己烯酮)靶的作用。更有效的途径是中断Barton乙烯碘化物的合成,然后氧化消除碘化物以有效地在靶标中建立烯酮官能团。双(环己烯酮)产物可用于合成lomaiviticin A本身。
更新日期:2020-07-22
中文翻译:

(-)-lomaiviticin A的双(环己烯酮)核心的合成
( - ) - Lomaiviticin A是一个复杂的Ç 2 -对称的细菌代谢物,其包括两个diazotetrahydrobenzo [ b ]芴(diazofluorene)残基和四个-2,6-二脱氧苷,α-大号-oleandrose和Ñ,ñ -二甲基- β-大号-吡咯烷胺。lomaiviticin A的两半通过单个碳-碳键定向的syn连接关于夹竹桃糖残基。尽管已经报道了合成lomaiviticin A的许多进展,包括糖苷配基的合成,但是尚未公开通往带有任何碳水化合物残基的双(环己烯酮)核心的途径。在这里,我们描述了一条带有两个α- L的lomaiviticin A核心结构的捷径-oleandrose残基。合成途径具有斯蒂勒偶联作用,形成靶标的碳-碳键,以及双重还原性转座,以在该键处建立正确的立体化学。开发了两种合成途径以阐明还原性转座产物对双(环己烯酮)靶的作用。更有效的途径是中断Barton乙烯碘化物的合成,然后氧化消除碘化物以有效地在靶标中建立烯酮官能团。双(环己烯酮)产物可用于合成lomaiviticin A本身。


























 京公网安备 11010802027423号
京公网安备 11010802027423号