当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
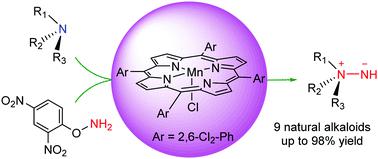
Direct preparation of unprotected aminimides (R3N+-NH-) from natural aliphatic tertiary alkaloids (R3N) by [Mn(TDCPP)Cl]-catalysed N-amination reaction.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0cc02934c Shilong Zhang 1 , Yungen Liu 1 , Fangrong Xing 2 , Chi-Ming Che 3
Chemical Communications ( IF 4.9 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0cc02934c Shilong Zhang 1 , Yungen Liu 1 , Fangrong Xing 2 , Chi-Ming Che 3
Affiliation
|
A panel of natural aliphatic tertiary alkaloids (R3N) were directly converted to R3N+–NH− (without the need to prepare protected aminimides R3N+–NR′− followed by deprotection) by [Mn(TDCPP)Cl]-catalysed N-amination reaction, with O-(2,4-dinitrophenyl)hydroxylamine as the nitrogen source, in up to 98% yields under mild reaction conditions.
中文翻译:

通过[Mn(TDCPP)Cl]催化的N胺化反应,从天然脂肪族叔生物碱(R3N)直接制备未保护的氨基酰亚胺(R3N + -NH-)。
天然脂肪族叔胺生物碱的面板(R 3 N)直接转化为R 3 Ñ + -NH - (无需制备保护的胺化酰亚胺- [R 3 Ñ + -NR' -接着脱保护)由[Mn(上TDCPP)氯在温和的反应条件下,以O-(2,4-二硝基苯基)羟胺为氮源,经[ - ]催化的N-胺化反应,收率高达98%。
更新日期:2020-08-11
中文翻译:

通过[Mn(TDCPP)Cl]催化的N胺化反应,从天然脂肪族叔生物碱(R3N)直接制备未保护的氨基酰亚胺(R3N + -NH-)。
天然脂肪族叔胺生物碱的面板(R 3 N)直接转化为R 3 Ñ + -NH - (无需制备保护的胺化酰亚胺- [R 3 Ñ + -NR' -接着脱保护)由[Mn(上TDCPP)氯在温和的反应条件下,以O-(2,4-二硝基苯基)羟胺为氮源,经[ - ]催化的N-胺化反应,收率高达98%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号