Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.jmgm.2020.107649 Sajedeh Sharifpour 1 , Sara Fakhraee 1 , Reza Behjatmanesh-Ardakani 1
|
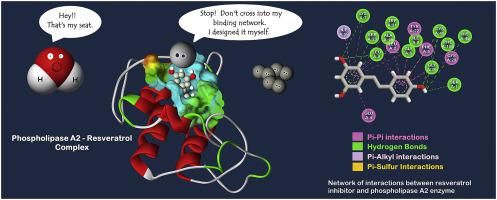
Phospholipase A2 (PLA2) is one of the enzymes involved in the development of cardiovascular diseases, vascular inflammation, risk of heart attacks, and strokes. This enzyme is responsible for catalyzing the hydrolytic cleavage of ester bonds of phospholipids in the biological pathway of inflammation. To prevent the undesired hydrolysis of phospholipids, the catalytic activity of PLA2 needs to be blocked. Resveratrol is a plant-derived polyphenol inhibitor, proven to have anti-inflammatory properties. However, there is still substantial ambiguity about its inhibitory function. The present study uncovers a detailed molecular mechanism behind the resveratrol action in inhibition of PLA2, by applying and comparing two 200-ns molecular dynamics simulations. The results of structural analyses revealed that the binding of resveratrol to PLA2 reduces the content of -sheets and increases a 5-helix to PLA2 structure, producing more folding and stability in protein. In the active site, the resveratrol is placed between the N-terminal -helix and the newly formed 5-helix through the hydrophobic interactions with ILE19 and LEU3 residues, as well as the hydrogen bond interactions. These interactions play the role of a network at the entrance of the enzyme active site and prevent the penetration of water molecules into the PLA2 cavity. A high occupancy hydrogen bonding has been identified between SER23 of the protein and hydroxyl group of resveratrol. Furthermore, the estimation of binding free energy verified the binding affinity of resveratrol is thermodynamically sufficient to be stably bounded to PLA2. It also proved that the van der Waals interactions, particularly hydrophobic interactions, have the most significant role in PLA2-resveratrol binding and stability. Overall, our results provide useful information on the stepwise mechanism of the inhibition of PLA2 enzyme by resveratrol, as a target for improving the pharmacological applications.
中文翻译:

白藜芦醇抑制磷脂酶A2机理的见解:广泛的分子动力学模拟和结合自由能计算。
磷脂酶A2(PLA2)是参与心血管疾病,血管炎症,心脏病发作和中风的酶之一。该酶负责在炎症的生物途径中催化磷脂的酯键的水解裂解。为了防止磷脂不希望的水解,需要阻止PLA2的催化活性。白藜芦醇是植物来源的多酚抑制剂,被证明具有抗炎特性。但是,其抑制功能仍然存在很大的歧义。本研究通过应用和比较两个200 ns的分子动力学模拟,揭示了白藜芦醇抑制PLA2背后的详细分子机制。-折叠并增加5螺旋至PLA2结构,从而在蛋白质中产生更多的折叠和稳定性。在活性部位,白藜芦醇位于N末端之间-螺旋和新形成的5-螺旋通过与ILE19和LEU3残基的疏水相互作用以及氢键相互作用而形成。这些相互作用在酶活性位点的入口处起到网络的作用,并防止水分子渗透到PLA2腔中。在蛋白质的SER23和白藜芦醇的羟基之间已发现高占用氢键。此外,结合自由能的估计证实了白藜芦醇的结合亲和力在热力学上足以稳定地结合至PLA2。还证明了范德华相互作用,特别是疏水相互作用,在PLA2-白藜芦醇的结合和稳定性中具有最重要的作用。总体,



























 京公网安备 11010802027423号
京公网安备 11010802027423号