Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical investigation of adsorption of graphene oxide at an interface between two immiscible electrolyte solutions
RSC Advances ( IF 3.9 ) Pub Date : 2020-7-8 , DOI: 10.1039/d0ra02560g Haiyan Qiu 1 , Tao Jiang 1 , Xiaoyuan Wang 1 , Lin Zhu 1 , Qingwei Wang 1 , Yun Zhao 1 , Jianjian Ge 2 , Yong Chen 1
RSC Advances ( IF 3.9 ) Pub Date : 2020-7-8 , DOI: 10.1039/d0ra02560g Haiyan Qiu 1 , Tao Jiang 1 , Xiaoyuan Wang 1 , Lin Zhu 1 , Qingwei Wang 1 , Yun Zhao 1 , Jianjian Ge 2 , Yong Chen 1
Affiliation
|
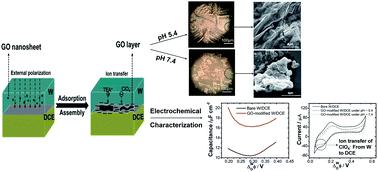
Graphene oxide (GO) has been recognized as an amphiphilic molecule or a soft colloidal particle with the ability to adsorb and assemble at the liquid/liquid (L/L) interface. However, most extant works concerning the adsorption behaviors of GO at the L/L interface have been limited to the non-polarized L/L interface. Here, we studied what would happen if GO nanosheets met with a polarizable L/L interface, namely an interface between two immiscible electrolyte solutions (ITIES). On one hand, the adsorption behavior of GO nanosheets at the L/L interface was electrochemically investigated firstly by using cyclic voltammetry (CV) and alternating current voltammetry (ACV). On the other hand, the influence of the adsorbed GO layers at the L/L interface on the ion transfer reactions was studied by employing ion-transfer voltammetry of TEA+ and ClO4− selected as probe ions. Capacitance measurements show that the interfacial capacitance increases greatly in the presence of GO nanosheets inside the aqueous phase, which can be attributed to the increases of interfacial corrugation and charge density induced by the parallel adsorption and assembly of GO at the L/L interface. In addition, it is found that the application of an interfacial potential difference by external polarization can promote the adsorption of GO at the L/L interface. Moreover, the ion-transfer voltammetric results further demonstrate that the GO layers formed at the interface can suppress the ion transfer reactions due to interfacial blocking and charge screening, as well as the hindrance effect induced by the GO layers. All the results with insights into the interfacial behavior of GO under polarization with an external electric field enable understanding the adsorption behavior of GO at the L/L interface more comprehensively.
中文翻译:

两种不混溶电解质溶液界面吸附氧化石墨烯的电化学研究
氧化石墨烯(GO)被认为是一种两亲分子或软胶体颗粒,具有在液/液(L/L)界面吸附和组装的能力。然而,大多数现存的关于 GO 在 L/L 界面上的吸附行为的工作仅限于非极化 L/L 界面。在这里,我们研究了如果 GO 纳米片遇到可极化的 L/L 界面,即两种不混溶电解质溶液 (ITIES) 之间的界面,会发生什么。一方面,首先使用循环伏安法(CV)和交流伏安法(ACV)对GO纳米片在L/L界面的吸附行为进行了电化学研究。另一方面,采用 TEA 的离子转移伏安法研究了 L/L 界面吸附的 GO 层对离子转移反应的影响。+和 ClO 4 -选作探针离子。电容测量表明,在水相中存在 GO 纳米片的情况下,界面电容显着增加,这可归因于 GO 在 L/L 界面的平行吸附和组装引起的界面波纹和电荷密度的增加。此外,发现通过外部极化施加界面电位差可以促进GO在L/L界面的吸附。此外,离子转移伏安法结果进一步表明,在界面处形成的 GO 层可以抑制由于界面阻挡和电荷屏蔽引起的离子转移反应,以及 GO 层引起的阻碍效应。
更新日期:2020-07-08
中文翻译:

两种不混溶电解质溶液界面吸附氧化石墨烯的电化学研究
氧化石墨烯(GO)被认为是一种两亲分子或软胶体颗粒,具有在液/液(L/L)界面吸附和组装的能力。然而,大多数现存的关于 GO 在 L/L 界面上的吸附行为的工作仅限于非极化 L/L 界面。在这里,我们研究了如果 GO 纳米片遇到可极化的 L/L 界面,即两种不混溶电解质溶液 (ITIES) 之间的界面,会发生什么。一方面,首先使用循环伏安法(CV)和交流伏安法(ACV)对GO纳米片在L/L界面的吸附行为进行了电化学研究。另一方面,采用 TEA 的离子转移伏安法研究了 L/L 界面吸附的 GO 层对离子转移反应的影响。+和 ClO 4 -选作探针离子。电容测量表明,在水相中存在 GO 纳米片的情况下,界面电容显着增加,这可归因于 GO 在 L/L 界面的平行吸附和组装引起的界面波纹和电荷密度的增加。此外,发现通过外部极化施加界面电位差可以促进GO在L/L界面的吸附。此外,离子转移伏安法结果进一步表明,在界面处形成的 GO 层可以抑制由于界面阻挡和电荷屏蔽引起的离子转移反应,以及 GO 层引起的阻碍效应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号