当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water Bridges Play a Key Role in Affinity and Selectivity for Malarial Protease Falcipain-2.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.jcim.0c00294 Jorge Enrique Hernández González 1 , Lilian Hernández Alvarez 1, 2 , Vitor B P Leite 1 , Pedro Geraldo Pascutti 3
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.jcim.0c00294 Jorge Enrique Hernández González 1 , Lilian Hernández Alvarez 1, 2 , Vitor B P Leite 1 , Pedro Geraldo Pascutti 3
Affiliation
|
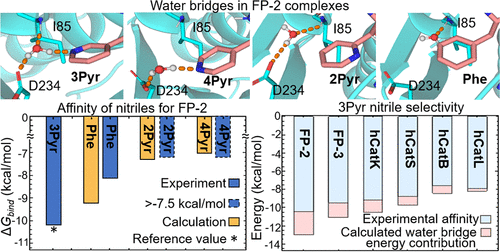
Falcipain-2 (FP-2) is hemoglobinase considered an attractive drug target of Plasmodium falciparum. Recently, it has been shown that peptidomimetic nitriles containing a 3-pyridyl (3Pyr) moiety at P2 display high affinity and selectivity for FP-2 with respect to human cysteine cathepsins (hCats), outperforming other P2-Pyr isomers and analogs. Further characterization demonstrated that certain P3 variants of these compounds possess micromolar inhibition of parasite growth in vitro and no cytotoxicity against human cell lines. However, the structural determinants underlying the selectivity of the 3Pyr-containing nitriles for FP-2 remain unknown. In this work, we conduct a thorough computational study combining MD simulations and free energy calculations to decipher the bases of the selectivity of the aforementioned nitriles. Our results reveal that water bridges involving the nitrogen and one carboxyl oxygen of I85 and D234 of FP-2, respectively, and the nitrogen of the neutral 3Pyr moiety, which are either less prevalent or nonexistent in the other complexes, explain the experimental activity profiles. The presence of crystallographic waters close to the bridging water positions in the studied proteases strongly supports the occurrence of such interactions. Overall, our findings suggest that selective FP-2 inhibitors can be designed by promoting water bridge formation at the bottom of the S2 subsite and/or by introducing complementary groups that displace the bridging water.
中文翻译:

水桥在疟疾蛋白酶Falcipain-2的亲和力和选择性中起关键作用。
Falcipain-2(FP-2)是血红蛋白酶,被认为是恶性疟原虫的有吸引力的药物靶标。最近,已经显示在P2处含有3-吡啶基(3Pyr)部分的拟肽腈相对于人半胱氨酸组织蛋白酶(hCats)显示出对FP-2的高亲和力和选择性,优于其他P2-Pyr异构体和类似物。进一步的表征表明,这些化合物的某些P3变体具有体外抑制寄生虫生长的微摩尔抑制作用而且对人细胞系无细胞毒性。但是,尚不清楚含3Pyr的腈对FP-2的选择性的结构决定因素。在这项工作中,我们进行了全面的计算研究,结合了MD模拟和自由能计算,以破译上述腈的选择性基础。我们的结果表明,水桥分别涉及FP-2的I85和D234的氮和一个羧基氧以及中性3Pyr部分的氮(在其他配合物中较不普遍或不存在),解释了实验活性图。在所研究的蛋白酶中靠近桥水位置的结晶水的存在强烈支持了这种相互作用的发生。总体,
更新日期:2020-07-07
中文翻译:

水桥在疟疾蛋白酶Falcipain-2的亲和力和选择性中起关键作用。
Falcipain-2(FP-2)是血红蛋白酶,被认为是恶性疟原虫的有吸引力的药物靶标。最近,已经显示在P2处含有3-吡啶基(3Pyr)部分的拟肽腈相对于人半胱氨酸组织蛋白酶(hCats)显示出对FP-2的高亲和力和选择性,优于其他P2-Pyr异构体和类似物。进一步的表征表明,这些化合物的某些P3变体具有体外抑制寄生虫生长的微摩尔抑制作用而且对人细胞系无细胞毒性。但是,尚不清楚含3Pyr的腈对FP-2的选择性的结构决定因素。在这项工作中,我们进行了全面的计算研究,结合了MD模拟和自由能计算,以破译上述腈的选择性基础。我们的结果表明,水桥分别涉及FP-2的I85和D234的氮和一个羧基氧以及中性3Pyr部分的氮(在其他配合物中较不普遍或不存在),解释了实验活性图。在所研究的蛋白酶中靠近桥水位置的结晶水的存在强烈支持了这种相互作用的发生。总体,



























 京公网安备 11010802027423号
京公网安备 11010802027423号