当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of Multisite Metal-Ligand Cooperativity on the Redox Activity of Noninnocent N2S2 Ligands.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-07-08 , DOI: 10.1021/acs.inorgchem.0c01353 Kyle D Spielvogel 1 , Javier A Luna 1 , Sydney M Loria 2 , Leah P Weisburn 2 , Nathan C Stumme 1 , Mark R Ringenberg 3 , Gummadi Durgaprasad 1 , Jason M Keith 2 , Scott K Shaw 1 , Scott R Daly 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-07-08 , DOI: 10.1021/acs.inorgchem.0c01353 Kyle D Spielvogel 1 , Javier A Luna 1 , Sydney M Loria 2 , Leah P Weisburn 2 , Nathan C Stumme 1 , Mark R Ringenberg 3 , Gummadi Durgaprasad 1 , Jason M Keith 2 , Scott K Shaw 1 , Scott R Daly 1
Affiliation
|
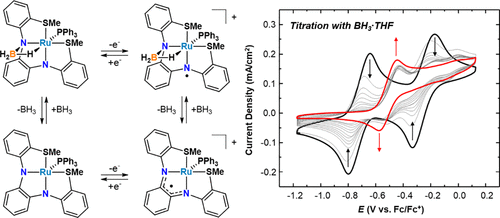
Metal–ligand cooperativity (MLC) relies on chemically reactive ligands to assist metals with small-molecule binding and activation, and it has facilitated unprecedented examples of catalysis with metal complexes. Despite growing interest in combining ligand-centered chemical and redox reactions for chemical transformations, there are few studies demonstrating how chemically engaging redox active ligands in MLC affects their electrochemical properties when bound to metals. Here we report stepwise changes in the redox activity of model Ru complexes as zero, one, and two BH3 molecules undergo MLC binding with a triaryl noninnocent N2S2 ligand derived from o-phenylenediamine (L1). A similar series of Ru complexes with a diaryl N2S2 ligand with ethylene substituted in place of phenylene (L2) is also described to evaluate the influence of the o-phenylenediamine subunit on redox activity and MLC. Cyclic voltammetry (CV) studies and density functional theory (DFT) calculations show that MLC attenuates ligand-centered redox activity in both series of complexes, but electron transfer is still achieved when only one of the two redox-active sites on the ligands is chemically engaged. The results demonstrate how incorporating more than one multifunctional reactive site could be an effective strategy for maintaining redox noninnocence in ligands that are also chemically reactive and competent for MLC.
中文翻译:

多部位金属-配体配合性对非纯N2S2配体氧化还原活性的影响。
金属-配体协同作用(MLC)依靠化学反应性配体来协助金属进行小分子结合和活化,它促进了金属配合物催化的空前实例。尽管对结合以配体为中心的化学和氧化还原反应进行化学转化的兴趣日益浓厚,但很少有研究证明MLC中的化学结合氧化还原活性配体与金属结合时如何影响其电化学性能。在这里,我们报告了模型Ru络合物的氧化还原活性的逐步变化,因为零,一和两个BH 3分子与衍生自邻苯二胺(L1的三芳基非纯N 2 S 2配体)进行MLC结合)。还描述了类似的一系列具有二芳基N 2 S 2配体的Ru配合物,其中乙烯取代了亚苯基(L2),以评估邻苯二胺亚基对氧化还原活性和MLC的影响。循环伏安法(CV)研究和密度泛函理论(DFT)计算表明,MLC减弱了这两种配合物中的以配体为中心的氧化还原活性,但是当配体上的两个氧化还原活性位中只有一个是化学反应时,仍能实现电子转移订婚。结果表明,结合多个以上的多功能反应位点如何成为一种有效的策略,可在具有化学反应性且能胜任MLC的配体中保持氧化还原非纯性。
更新日期:2020-08-03
中文翻译:

多部位金属-配体配合性对非纯N2S2配体氧化还原活性的影响。
金属-配体协同作用(MLC)依靠化学反应性配体来协助金属进行小分子结合和活化,它促进了金属配合物催化的空前实例。尽管对结合以配体为中心的化学和氧化还原反应进行化学转化的兴趣日益浓厚,但很少有研究证明MLC中的化学结合氧化还原活性配体与金属结合时如何影响其电化学性能。在这里,我们报告了模型Ru络合物的氧化还原活性的逐步变化,因为零,一和两个BH 3分子与衍生自邻苯二胺(L1的三芳基非纯N 2 S 2配体)进行MLC结合)。还描述了类似的一系列具有二芳基N 2 S 2配体的Ru配合物,其中乙烯取代了亚苯基(L2),以评估邻苯二胺亚基对氧化还原活性和MLC的影响。循环伏安法(CV)研究和密度泛函理论(DFT)计算表明,MLC减弱了这两种配合物中的以配体为中心的氧化还原活性,但是当配体上的两个氧化还原活性位中只有一个是化学反应时,仍能实现电子转移订婚。结果表明,结合多个以上的多功能反应位点如何成为一种有效的策略,可在具有化学反应性且能胜任MLC的配体中保持氧化还原非纯性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号