当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
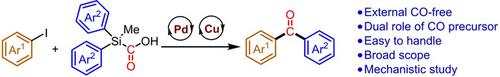
Silylcarboxylic Acids as Bifunctional Reagents: Application in Palladium‐Catalyzed External‐CO‐Free Carbonylative Cross‐Coupling Reactions
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-07-07 , DOI: 10.1002/adsc.202000586 Xiong Li 1 , Jie Xu 1 , Yue Li 1 , Søren Kramer 2 , Troels Skrydstrup 3 , Zhong Lian 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-07-07 , DOI: 10.1002/adsc.202000586 Xiong Li 1 , Jie Xu 1 , Yue Li 1 , Søren Kramer 2 , Troels Skrydstrup 3 , Zhong Lian 1
Affiliation
|
A palladium‐catalyzed external‐CO‐free carbonylative Hiyama‐Denmark cross‐coupling reaction is presented. The introduction of silylcarboxylic acids as bifunctional reagents (CO and nucleophile source) avoids the need for external gaseous CO and a silylarene coupling partner. The transformation features high functional group tolerance and it is successful with electron‐rich, ‐neutral, and ‐poor aryl iodides. Stoichiometric studies and control experiments provide insight into the reaction mechanism and support the hypothesized dual role of silylcarboxylic acids.
中文翻译:

甲硅烷基羧酸作为双功能试剂:在钯催化的外部无CO羰基交叉偶联反应中的应用
提出了钯催化的外部无CO羰基的Hiyama-Denmark交叉偶联反应。甲硅烷基羧酸作为双功能试剂(CO和亲核试剂来源)的引入避免了对外部气体CO和甲硅烷基芳烃偶联伙伴的需求。该转变具有很高的官能团耐受性,并且可以成功地用于富电子,中性和差的芳基碘化物。化学计量研究和对照实验提供了对反应机理的深入了解并支持了甲硅烷基羧酸的双重作用。
更新日期:2020-07-07
中文翻译:

甲硅烷基羧酸作为双功能试剂:在钯催化的外部无CO羰基交叉偶联反应中的应用
提出了钯催化的外部无CO羰基的Hiyama-Denmark交叉偶联反应。甲硅烷基羧酸作为双功能试剂(CO和亲核试剂来源)的引入避免了对外部气体CO和甲硅烷基芳烃偶联伙伴的需求。该转变具有很高的官能团耐受性,并且可以成功地用于富电子,中性和差的芳基碘化物。化学计量研究和对照实验提供了对反应机理的深入了解并支持了甲硅烷基羧酸的双重作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号