当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Overexpression of ER Protein Processing and Apoptosis Regulator Genes in Human Embryonic Kidney 293 Cells Improves Gene Therapy Vectors Production.
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-07-08 , DOI: 10.1002/biot.201900562 Ana S Formas-Oliveira 1, 2 , João S Basílio 1, 2 , Ana F Rodrigues 1, 2 , Ana S Coroadinha 1, 2, 3
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-07-08 , DOI: 10.1002/biot.201900562 Ana S Formas-Oliveira 1, 2 , João S Basílio 1, 2 , Ana F Rodrigues 1, 2 , Ana S Coroadinha 1, 2, 3
Affiliation
|
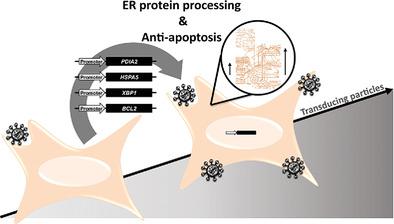
Gammaretroviral and lentiviral vectors (γ‐RV and LV) are among the most used vectors in gene therapy. Currently, human embryonic kidney (HEK) 293 cells, the manufacture platform of choice for these vectors, provide low transducing particle yields, challenging their therapeutic applications and commercialization. This work studies metabolic pathways, focusing on endoplasmic reticulum (ER) protein processing and anti‐apoptotic mechanisms, influencing vector productivity in HEK 293 cell substrates. To that end, four candidate genes—protein disulfide isomerase family A member 2 gene, heat shock protein family A (Hsp70) member 5 gene, X‐box binding protein 1 gene (ER protein processing), and B‐cell lymphoma 2 protein gene (anti‐apoptotic)—are individually stably expressed in the cells. How their overexpression level influence vector yields is analyzed by establishing cell populations with incremental genomic copies of each. γ‐RV volumetric productivity increases up to 97% when overexpressing ER protein processing genes. LV volumetric production increases 53% when overexpressing the anti‐apoptotic gene. Improvements are associated with higher cell specific productivities and dependent on gene overexpression level, highlighting the importance of fine‐tuning gene expression. Overall, this work discloses gene engineering targets enabling efficient gene therapy product manufacture showing that ER protein processing and anti‐apoptotic pathways are pivotal to producer cell performance.
中文翻译:

人胚肾293细胞中ER蛋白加工和凋亡调节基因的过表达改善了基因治疗载体的生产。
γ逆转录病毒和慢病毒载体(γ‐RV和LV)是基因治疗中使用最广泛的载体。目前,人类胚胎肾(HEK)293细胞(这些载体的选择制造平台)提供的转导颗粒产量低,对它们的治疗应用和商业化提出了挑战。这项工作研究代谢途径,重点是内质网(ER)蛋白质加工和抗凋亡机制,影响HEK 293细胞底物中的载体生产力。为此,有四个候选基因:蛋白二硫键异构酶家族A成员2基因,热休克蛋白A家族(Hsp70)成员5基因,X-box结合蛋白1基因(ER蛋白加工)和B细胞淋巴瘤2蛋白基因(抗凋亡)-在细胞中稳定表达。通过建立具有递增的基因组拷贝的细胞群体,分析它们的过表达水平如何影响载体产量。当过表达ER蛋白加工基因时,γ-RV的容积生产率提高高达97%。当过表达抗凋亡基因时,左室容积增加53%。改善与更高的细胞特异性生产力相关,并取决于基因的过表达水平,这凸显了微调基因表达的重要性。总的来说,这项工作公开了基因工程靶标,可实现高效基因治疗产品的生产,表明ER蛋白加工和抗凋亡途径对生产细胞的性能至关重要。当过表达ER蛋白加工基因时,γ-RV的容积生产率提高高达97%。当过表达抗凋亡基因时,左室容积增加53%。改善与更高的细胞特异性生产力相关,并取决于基因的过表达水平,这凸显了微调基因表达的重要性。总的来说,这项工作公开了基因工程靶标,可实现高效基因治疗产品的生产,表明ER蛋白加工和抗凋亡途径对生产细胞的性能至关重要。当过表达ER蛋白加工基因时,γ-RV的容积生产率提高高达97%。当过表达抗凋亡基因时,左室容积增加53%。改善与更高的细胞特异性生产力相关,并取决于基因过表达水平,这突出说明了微调基因表达的重要性。总的来说,这项工作公开了基因工程靶标,可实现高效基因治疗产品的生产,表明ER蛋白加工和抗凋亡途径对生产细胞的性能至关重要。
更新日期:2020-09-02
中文翻译:

人胚肾293细胞中ER蛋白加工和凋亡调节基因的过表达改善了基因治疗载体的生产。
γ逆转录病毒和慢病毒载体(γ‐RV和LV)是基因治疗中使用最广泛的载体。目前,人类胚胎肾(HEK)293细胞(这些载体的选择制造平台)提供的转导颗粒产量低,对它们的治疗应用和商业化提出了挑战。这项工作研究代谢途径,重点是内质网(ER)蛋白质加工和抗凋亡机制,影响HEK 293细胞底物中的载体生产力。为此,有四个候选基因:蛋白二硫键异构酶家族A成员2基因,热休克蛋白A家族(Hsp70)成员5基因,X-box结合蛋白1基因(ER蛋白加工)和B细胞淋巴瘤2蛋白基因(抗凋亡)-在细胞中稳定表达。通过建立具有递增的基因组拷贝的细胞群体,分析它们的过表达水平如何影响载体产量。当过表达ER蛋白加工基因时,γ-RV的容积生产率提高高达97%。当过表达抗凋亡基因时,左室容积增加53%。改善与更高的细胞特异性生产力相关,并取决于基因的过表达水平,这凸显了微调基因表达的重要性。总的来说,这项工作公开了基因工程靶标,可实现高效基因治疗产品的生产,表明ER蛋白加工和抗凋亡途径对生产细胞的性能至关重要。当过表达ER蛋白加工基因时,γ-RV的容积生产率提高高达97%。当过表达抗凋亡基因时,左室容积增加53%。改善与更高的细胞特异性生产力相关,并取决于基因的过表达水平,这凸显了微调基因表达的重要性。总的来说,这项工作公开了基因工程靶标,可实现高效基因治疗产品的生产,表明ER蛋白加工和抗凋亡途径对生产细胞的性能至关重要。当过表达ER蛋白加工基因时,γ-RV的容积生产率提高高达97%。当过表达抗凋亡基因时,左室容积增加53%。改善与更高的细胞特异性生产力相关,并取决于基因过表达水平,这突出说明了微调基因表达的重要性。总的来说,这项工作公开了基因工程靶标,可实现高效基因治疗产品的生产,表明ER蛋白加工和抗凋亡途径对生产细胞的性能至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号