Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Genetic driver mutations introduced in identical cell-of-origin in murine glioblastoma reveal distinct immune landscapes but similar response to checkpoint blockade.
Glia ( IF 6.2 ) Pub Date : 2020-07-08 , DOI: 10.1002/glia.23883 Zhihong Chen 1, 2, 3 , Cameron J Herting 3, 4 , James L Ross 3, 5 , Ben Gabanic 3 , Montse Puigdelloses Vallcorba 3, 6, 7, 8 , Frank Szulzewsky 9 , Megan L Wojciechowicz 10 , Patrick J Cimino 11 , Ravesanker Ezhilarasan 12, 13 , Erik P Sulman 12, 13 , Mingyao Ying 14 , Avi Ma'ayan 10 , Renee D Read 15, 16 , Dolores Hambardzumyan 1, 2, 3
Glia ( IF 6.2 ) Pub Date : 2020-07-08 , DOI: 10.1002/glia.23883 Zhihong Chen 1, 2, 3 , Cameron J Herting 3, 4 , James L Ross 3, 5 , Ben Gabanic 3 , Montse Puigdelloses Vallcorba 3, 6, 7, 8 , Frank Szulzewsky 9 , Megan L Wojciechowicz 10 , Patrick J Cimino 11 , Ravesanker Ezhilarasan 12, 13 , Erik P Sulman 12, 13 , Mingyao Ying 14 , Avi Ma'ayan 10 , Renee D Read 15, 16 , Dolores Hambardzumyan 1, 2, 3
Affiliation
|
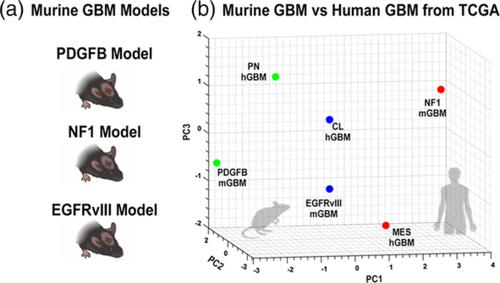
Glioblastoma (GBM) is the most aggressive primary brain tumor. In addition to being genetically heterogeneous, GBMs are also immunologically heterogeneous. However, whether the differences in immune microenvironment are driven by genetic driver mutation is unexplored. By leveraging the versatile RCAS/tv‐a somatic gene transfer system, we establish a mouse model for Classical GBM by introducing EGFRvIII expression in Nestin ‐positive neural stem/progenitor cells in adult mice. Along with our previously published Nf1 ‐silenced and PDGFB‐overexpressing models, we investigate the immune microenvironments of the three models of human GBM subtypes by unbiased multiplex profiling. We demonstrate that both the quantity and composition of the microenvironmental myeloid cells are dictated by the genetic driver mutations, closely mimicking what was observed in human GBM subtypes. These myeloid cells express high levels of the immune checkpoint protein PD‐L1; however, PD‐L1 targeted therapies alone or in combination with irradiation are unable to increase the survival time of tumor‐bearing mice regardless of the driver mutations, reflecting the outcomes of recent human trials. Together, these results highlight the critical utility of immunocompetent mouse models for preclinical studies of GBM, making these models indispensable tools for understanding the resistance mechanisms of immune checkpoint blockade in GBM and immune cell‐targeting drug discovery.
中文翻译:

在小鼠胶质母细胞瘤的相同细胞源中引入的基因驱动突变揭示了不同的免疫景观,但对检查点封锁的反应相似。
胶质母细胞瘤(GBM)是最具侵袭性的原发性脑肿瘤。除了遗传异质性之外,GBMs 还具有免疫异质性。然而,免疫微环境的差异是否由基因驱动突变驱动尚待探索。通过利用多功能的 RCAS/ tv-a体细胞基因转移系统,我们通过在成年小鼠的巢蛋白阳性神经干/祖细胞中引入 EGFRvIII 表达来建立经典 GBM 的小鼠模型。连同我们之前发布的Nf1-沉默和 PDGFB 过表达模型,我们通过无偏多重分析研究了三种人类 GBM 亚型模型的免疫微环境。我们证明微环境骨髓细胞的数量和组成都由基因驱动突变决定,与在人类 GBM 亚型中观察到的非常相似。这些骨髓细胞表达高水平的免疫检查点蛋白 PD-L1;然而,无论驱动突变如何,单独使用 PD-L1 靶向治疗或与辐射联合使用都无法增加荷瘤小鼠的存活时间,这反映了最近人体试验的结果。总之,这些结果突出了免疫活性小鼠模型在 GBM 临床前研究中的关键效用,
更新日期:2020-08-10
中文翻译:

在小鼠胶质母细胞瘤的相同细胞源中引入的基因驱动突变揭示了不同的免疫景观,但对检查点封锁的反应相似。
胶质母细胞瘤(GBM)是最具侵袭性的原发性脑肿瘤。除了遗传异质性之外,GBMs 还具有免疫异质性。然而,免疫微环境的差异是否由基因驱动突变驱动尚待探索。通过利用多功能的 RCAS/ tv-a体细胞基因转移系统,我们通过在成年小鼠的巢蛋白阳性神经干/祖细胞中引入 EGFRvIII 表达来建立经典 GBM 的小鼠模型。连同我们之前发布的Nf1-沉默和 PDGFB 过表达模型,我们通过无偏多重分析研究了三种人类 GBM 亚型模型的免疫微环境。我们证明微环境骨髓细胞的数量和组成都由基因驱动突变决定,与在人类 GBM 亚型中观察到的非常相似。这些骨髓细胞表达高水平的免疫检查点蛋白 PD-L1;然而,无论驱动突变如何,单独使用 PD-L1 靶向治疗或与辐射联合使用都无法增加荷瘤小鼠的存活时间,这反映了最近人体试验的结果。总之,这些结果突出了免疫活性小鼠模型在 GBM 临床前研究中的关键效用,


























 京公网安备 11010802027423号
京公网安备 11010802027423号