当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atomic-Scale Studies of Fe3 O4 (001) and TiO2 (110) Surfaces Following Immersion in CO2 -Acidified Water.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-08-03 , DOI: 10.1002/cphc.202000471 Francesca Mirabella 1 , Jan Balajka 1, 2 , Jiri Pavelec 1 , Markus Göbel 1 , Florian Kraushofer 1 , Michael Schmid 1 , Gareth S Parkinson 1 , Ulrike Diebold 1
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-08-03 , DOI: 10.1002/cphc.202000471 Francesca Mirabella 1 , Jan Balajka 1, 2 , Jiri Pavelec 1 , Markus Göbel 1 , Florian Kraushofer 1 , Michael Schmid 1 , Gareth S Parkinson 1 , Ulrike Diebold 1
Affiliation
|
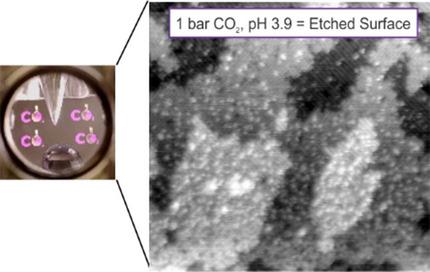
Difficulties associated with the integration of liquids into a UHV environment make surface‐science style studies of mineral dissolution particularly challenging. Recently, we developed a novel experimental setup for the UHV‐compatible dosing of ultrapure liquid water and studied its interaction with TiO2 and Fe3O4 surfaces. Herein, we describe a simple approach to vary the pH through the partial pressure of CO2 ( ) in the surrounding vacuum chamber and use this to study how these surfaces react to an acidic solution. The TiO2(110) surface is unaffected by the acidic solution, except for a small amount of carbonaceous contamination. The Fe3O4(001)‐(
) in the surrounding vacuum chamber and use this to study how these surfaces react to an acidic solution. The TiO2(110) surface is unaffected by the acidic solution, except for a small amount of carbonaceous contamination. The Fe3O4(001)‐( ×
× )R45° surface begins to dissolve at a pH 4.0–3.9 (
)R45° surface begins to dissolve at a pH 4.0–3.9 ( =0.8–1 bar) and, although it is significantly roughened, the atomic‐scale structure of the Fe3O4(001) surface layer remains visible in scanning tunneling microscopy (STM) images. X‐ray photoelectron spectroscopy (XPS) reveals that the surface is chemically reduced and contains a significant accumulation of bicarbonate (HCO3−) species. These observations are consistent with Fe(II) being extracted by bicarbonate ions, leading to dissolved iron bicarbonate complexes (Fe(HCO3)2), which precipitate onto the surface when the water evaporates.
=0.8–1 bar) and, although it is significantly roughened, the atomic‐scale structure of the Fe3O4(001) surface layer remains visible in scanning tunneling microscopy (STM) images. X‐ray photoelectron spectroscopy (XPS) reveals that the surface is chemically reduced and contains a significant accumulation of bicarbonate (HCO3−) species. These observations are consistent with Fe(II) being extracted by bicarbonate ions, leading to dissolved iron bicarbonate complexes (Fe(HCO3)2), which precipitate onto the surface when the water evaporates.
中文翻译:

Fe3 O4 (001) 和 TiO2 (110) 表面浸入 CO2 酸化水中后的原子尺度研究。
将液体整合到特高压环境中的困难使得矿物溶解的表面科学类型研究特别具有挑战性。最近,我们开发了一种新颖的实验装置,用于超纯液态水的 UHV 兼容计量,并研究了其与 TiO 2和 Fe 3 O 4表面的相互作用。 在此,我们描述了一种通过周围真空室中CO 2 ( )分压来改变 pH 值的简单方法,并用它来研究这些表面如何与酸性溶液反应。除少量碳质污染外, TiO 2 (110)表面未受酸性溶液的影响。Fe 3 O 4 (001)-(
TiO 2 (110)表面未受酸性溶液的影响。Fe 3 O 4 (001)-(  ×
×  )R45° 表面在 pH 4.0–3.9 (=0.8–1 bar) 时开始溶解,尽管表面明显粗糙,但 Fe 3 O 4
)R45° 表面在 pH 4.0–3.9 (=0.8–1 bar) 时开始溶解,尽管表面明显粗糙,但 Fe 3 O 4 的原子级结构(001) 表面层在扫描隧道显微镜 (STM) 图像中仍然可见。X射线光电子能谱(XPS)显示表面经过化学还原,并且含有大量碳酸氢盐(HCO 3 -)物质的积累。这些观察结果与碳酸氢根离子萃取 Fe(II) 一致,导致溶解的碳酸氢铁复合物 (Fe(HCO 3 ) 2 ),当水蒸发时沉淀到表面。
的原子级结构(001) 表面层在扫描隧道显微镜 (STM) 图像中仍然可见。X射线光电子能谱(XPS)显示表面经过化学还原,并且含有大量碳酸氢盐(HCO 3 -)物质的积累。这些观察结果与碳酸氢根离子萃取 Fe(II) 一致,导致溶解的碳酸氢铁复合物 (Fe(HCO 3 ) 2 ),当水蒸发时沉淀到表面。
更新日期:2020-08-03
 ) in the surrounding vacuum chamber and use this to study how these surfaces react to an acidic solution. The TiO2(110) surface is unaffected by the acidic solution, except for a small amount of carbonaceous contamination. The Fe3O4(001)‐(
) in the surrounding vacuum chamber and use this to study how these surfaces react to an acidic solution. The TiO2(110) surface is unaffected by the acidic solution, except for a small amount of carbonaceous contamination. The Fe3O4(001)‐( ×
× )R45° surface begins to dissolve at a pH 4.0–3.9 (
)R45° surface begins to dissolve at a pH 4.0–3.9 ( =0.8–1 bar) and, although it is significantly roughened, the atomic‐scale structure of the Fe3O4(001) surface layer remains visible in scanning tunneling microscopy (STM) images. X‐ray photoelectron spectroscopy (XPS) reveals that the surface is chemically reduced and contains a significant accumulation of bicarbonate (HCO3−) species. These observations are consistent with Fe(II) being extracted by bicarbonate ions, leading to dissolved iron bicarbonate complexes (Fe(HCO3)2), which precipitate onto the surface when the water evaporates.
=0.8–1 bar) and, although it is significantly roughened, the atomic‐scale structure of the Fe3O4(001) surface layer remains visible in scanning tunneling microscopy (STM) images. X‐ray photoelectron spectroscopy (XPS) reveals that the surface is chemically reduced and contains a significant accumulation of bicarbonate (HCO3−) species. These observations are consistent with Fe(II) being extracted by bicarbonate ions, leading to dissolved iron bicarbonate complexes (Fe(HCO3)2), which precipitate onto the surface when the water evaporates.
中文翻译:

Fe3 O4 (001) 和 TiO2 (110) 表面浸入 CO2 酸化水中后的原子尺度研究。
将液体整合到特高压环境中的困难使得矿物溶解的表面科学类型研究特别具有挑战性。最近,我们开发了一种新颖的实验装置,用于超纯液态水的 UHV 兼容计量,并研究了其与 TiO 2和 Fe 3 O 4表面的相互作用。 在此,我们描述了一种通过周围真空室中CO 2 ( )分压来改变 pH 值的简单方法,并用它来研究这些表面如何与酸性溶液反应。除少量碳质污染外,
 TiO 2 (110)表面未受酸性溶液的影响。Fe 3 O 4 (001)-(
TiO 2 (110)表面未受酸性溶液的影响。Fe 3 O 4 (001)-(  ×
×  )R45° 表面在 pH 4.0–3.9 (=0.8–1 bar) 时开始溶解,尽管表面明显粗糙,但 Fe 3 O 4
)R45° 表面在 pH 4.0–3.9 (=0.8–1 bar) 时开始溶解,尽管表面明显粗糙,但 Fe 3 O 4 的原子级结构(001) 表面层在扫描隧道显微镜 (STM) 图像中仍然可见。X射线光电子能谱(XPS)显示表面经过化学还原,并且含有大量碳酸氢盐(HCO 3 -)物质的积累。这些观察结果与碳酸氢根离子萃取 Fe(II) 一致,导致溶解的碳酸氢铁复合物 (Fe(HCO 3 ) 2 ),当水蒸发时沉淀到表面。
的原子级结构(001) 表面层在扫描隧道显微镜 (STM) 图像中仍然可见。X射线光电子能谱(XPS)显示表面经过化学还原,并且含有大量碳酸氢盐(HCO 3 -)物质的积累。这些观察结果与碳酸氢根离子萃取 Fe(II) 一致,导致溶解的碳酸氢铁复合物 (Fe(HCO 3 ) 2 ),当水蒸发时沉淀到表面。


























 京公网安备 11010802027423号
京公网安备 11010802027423号