Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.jmgm.2020.107668 Saira Sajjad 1 , Akhtar Ali 1 , Tariq Mahmood 1 , Khurshid Ayub 1
|
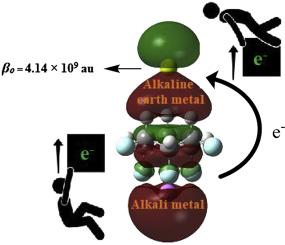
Alkaline earthide is a well-known class of the excess electron compounds with potential applications as NLO materials. In this study, all-cis-1,2,3,4,5,6-hexafluor-ocyclohexane C6H6F6 (1), a high polarized complexant having the largest dipole moment (6.2D) among all the known aliphatic hydrocarbons, is selected as a suitable molecule for designing a new series of excess electron molecules, alkaline-earthides. Geometric, thermodynamic and electronic properties of A-1-AE (A = Li, Na, K and AE = Be, Mg, Ca) are studied at M06-2X/6-31+G(d,p) level of theory. More specifically, alkaline-earthide nature is confirmed by distribution of densities in HOMOs, VIE values and NBO analysis. The alkaline earthide possess moderate complexation energies (−6.56 to −14.19 kcal/mol), which demonstrate their thermodynamic stability. These alkaline earthides possess very small excitation energies (0.54–1.64 eV) and very large first hyperpolarizabilities (up to 4.14 × 109 au). The higher hyperpolarizabilities values are attributed to the presence of excess electrons on alkaline earth metals which is confirmed through the partial density of states (PDOS) analysis. The hyperpolarizabilities are rationalized through two level model approach. Large hyperpolarizability values illustrate that the alkaline-earthides are a very promising entry into excess electron compounds.
中文翻译:

钠和钾作为过量电子源,具有出色的NLO响应的Janus碱土金属;第一原理研究。
碱土金属是一类众所周知的过量电子化合物,具有作为NLO材料的潜在应用。在这项研究中,全顺式-1,2,3,4,5,6-六氟-环己烷C 6 H 6 F 6(1),一种高极化的络合物,在所有已知的偶极矩中最大(6.2D)选择脂族烃作为合适的分子,以设计一系列新的过量电子分子碱土金属。A- 1的几何,热力学和电子性质在M06-2X / 6-31 + G(d,p)的理论水平上研究了-AE(A = Li,Na,K和AE = Be,Mg,Ca)。更具体地说,碱土金属的性质可以通过HOMO中的密度分布,VIE值和NBO分析得到证实。碱土金属具有中等的络合能(-6.56至-14.19 kcal / mol),显示出其热力学稳定性。这些碱土金属具有非常小的激发能(0.54–1.64 eV)和非常大的第一超极化率(高达4.14×10 9)au)。较高的超极化率值归因于碱土金属上过量电子的存在,这通过状态部分密度(PDOS)分析得到证实。超极化率通过两级模型方法得以合理化。大的超极化率值表明,碱土类是进入过量电子化合物中非常有希望的进入途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号