Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.jmgm.2020.107672 George Baffour Pipim 1 , Richard Tia 1 , Evans Adei 1
|
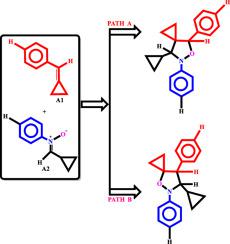
The biomedical importance of spirocyclopropane isoxazolidine derivatives is widely known. The 1,3-dipolar cycloaddition (1,3-DC) of C-cyclopropyl-N-phenylnitrone derivative and benzylidenecyclopropane derivatives leading to the formation of 5- and 4-spirocyclopropane isoxazolidines derivatives have been studied using density functional theory (DFT) at M06–2X/6-311G (d,p) level of theory. An extensive exploration of the potential energy surface shows that the 1,3-dipole adds across the dipolarophile via an asynchronous concerted mechanism. While electron-donating groups (EDGs) on the benzylidenecyclopropane favor the formation of the 4-spirocyclopropane isomer, electron-withdrawing groups (EWGs) favor the reaction channels that furnish the 5-spirocyclopropane isoxazolidine isomer. Both EWDs and EDGs on the 1,3-dipole favor the formation of the 5-spirocyclopropane isoxazolidine isomer. Irrespective of the electronic nature of substituents on the C-cyclopropyl-N-phenylnitrone, the reaction channels that regioselectively lead to the formation of the 5-spirocyclopropane isoxazolidine isomer are favored. In all reactions considered, the channels that selectively lead to the formation of the cis-diastereoisomers proceed with lower activation barriers than the trans-diastereoisomers. In all cases, the observed selectivities in the title reaction are kinetically controlled.
中文翻译:

研究C-环丙基-N-苯基硝酮衍生物和亚苄基环丙烷衍生物的1,3-偶极环加成反应的区域,立体和对映选择性:一项DFT研究。
螺环丙烷异恶唑烷衍生物的生物医学重要性是众所周知的。C-环丙基-N的1,3-偶极环加成(1,3-DC)在密度为M06–2X / 6-311G(d,p)的理论下,使用密度泛函理论(DFT)研究了导致5和4螺环丙烷异恶唑烷衍生物形成的-苯基硝酮衍生物和亚苄基环丙烷衍生物。对势能面的广泛研究表明,1,3-偶极子通过异步协同机制跨亲极性子增加。尽管亚苄基环丙烷上的给电子基团(EDG)有助于形成4-螺环丙烷异构体,而吸电子基团(EWG)则有利于提供5-螺环丙烷异恶唑烷异构体的反应通道。1,3-偶极子上的EWD和EDG都倾向于形成5-螺环丙烷异恶唑烷异构体。与C上取代基的电子性质无关-环丙基-N-苯基硝基,优选区域选择性地导致5-螺环丙烷异恶唑烷异构体形成的反应通道。在所有考虑的反应中,选择性地导致顺式-非对映异构体形成的通道比反式-非对映异构体具有更低的活化障碍。在所有情况下,标题反应中观察到的选择性是动力学控制的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号