当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigating the interaction between three perfluorinated carboxylic acids and the G protein-coupled estrogen receptor: spectroscopic analyses and computational simulations.
Analytical Methods ( IF 3.1 ) Pub Date : 2020-07-07 , DOI: 10.1039/d0ay01052a Li Yong 1 , Manting Huang , Yuchen Wei , Jie Xu , Zhongsheng Yi
Analytical Methods ( IF 3.1 ) Pub Date : 2020-07-07 , DOI: 10.1039/d0ay01052a Li Yong 1 , Manting Huang , Yuchen Wei , Jie Xu , Zhongsheng Yi
Affiliation
|
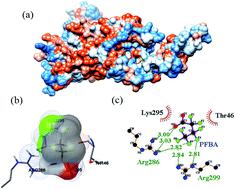
In this paper, perfluorinated compounds (PFCs), such as perfluorobutyric acid (PFBA), perfluorooctanoic acid (PFOA) and perfluorododecanoic acid (PFDoA), were selected as typical representatives of perfluorinated carboxylic acids (PFCAs) to study the effects of PFCAs on the G protein-coupled estrogen receptor (GPER). The interaction mechanism of the three types of PFCAs with the GPER was investigated using steady-state fluorescence spectroscopy, ultraviolet-visible spectroscopy, three-dimensional fluorescence spectroscopy, and Fourier transform infrared spectroscopy combined with molecular docking and molecular dynamics simulations. Among these techniques, steady-state fluorescence and ultraviolet-visible spectroscopic analyses showed that PFBA, PFOA and PFDoA quenched the endogenous GPER fluorescence by combined dynamic and static quenching and non-radiative energy transfer. The binding constants (Ka) of PFCAs on the GPER were all larger than 105 L mol−1, indicating that their affinity for the GPER was strong. Fourier transform infrared spectroscopy and three-dimensional fluorescence showed that the secondary structure of the GPER changed after binding to PFCAs. Thermodynamic analysis showed ΔG < 0, which indicated that the interaction between the GPER and PFCAs was spontaneous. For the binding of PFBA and PFOA to the GPER, ΔH > 0 and ΔS > 0, indicating that the interaction was mainly driven by hydrophobic forces; for the binding of PFDoA to the GPER, ΔH < 0 and ΔS < 0, suggesting that van der Waals force and hydrogen bonding were the main interaction forces. Molecular dynamics simulations suggested that the stability of the GPER–PFCA complexes was higher than that of the free GPER, and also that the structure and hydrophobicity of the GPER changed after binding to PFCAs. Molecular docking analysis showed that all three PFCAs could form hydrogen bonds with the GPER, which improved the stability of the complex.
中文翻译:

研究三种全氟羧酸与 G 蛋白偶联雌激素受体之间的相互作用:光谱分析和计算模拟。
本文选取全氟丁酸 (PFBA)、全氟辛酸 (PFOA) 和全氟十二烷酸 (PFDoA) 等全氟化合物 (PFCs) 作为全氟羧酸 (PFCAs) 的典型代表, 研究 PFCAs 对G 蛋白偶联雌激素受体 (GPER)。采用稳态荧光光谱、紫外-可见光谱、三维荧光光谱和傅里叶变换红外光谱结合分子对接和分子动力学模拟研究了三种PFCAs与GPER的相互作用机制。在这些技术中,稳态荧光和紫外-可见光谱分析表明,PFBA、PFOA 和 PFDoA 通过结合动态和静态猝灭和非辐射能量转移来猝灭内源性 GPER 荧光。绑定常量 (GPER上PFCA的Ka)均大于10 5 L mol -1,表明它们对GPER的亲和力很强。傅里叶变换红外光谱和三维荧光表明,GPER 的二级结构在与 PFCA 结合后发生了变化。热力学分析显示 Δ G < 0,这表明 GPER 和 PFCA 之间的相互作用是自发的。对于 PFBA 和 PFOA 与 GPER 的结合,Δ H > 0 和 Δ S > 0,表明相互作用主要由疏水力驱动;对于 PFDoA 与 GPER 的结合,Δ H < 0 和 Δ S< 0,表明范德华力和氢键是主要的相互作用力。分子动力学模拟表明,GPER-PFCA 复合物的稳定性高于游离 GPER,并且 GPER 的结构和疏水性在与 PFCA 结合后发生了变化。分子对接分析表明,三种PFCA都可以与GPER形成氢键,提高了复合物的稳定性。
更新日期:2020-08-14
中文翻译:

研究三种全氟羧酸与 G 蛋白偶联雌激素受体之间的相互作用:光谱分析和计算模拟。
本文选取全氟丁酸 (PFBA)、全氟辛酸 (PFOA) 和全氟十二烷酸 (PFDoA) 等全氟化合物 (PFCs) 作为全氟羧酸 (PFCAs) 的典型代表, 研究 PFCAs 对G 蛋白偶联雌激素受体 (GPER)。采用稳态荧光光谱、紫外-可见光谱、三维荧光光谱和傅里叶变换红外光谱结合分子对接和分子动力学模拟研究了三种PFCAs与GPER的相互作用机制。在这些技术中,稳态荧光和紫外-可见光谱分析表明,PFBA、PFOA 和 PFDoA 通过结合动态和静态猝灭和非辐射能量转移来猝灭内源性 GPER 荧光。绑定常量 (GPER上PFCA的Ka)均大于10 5 L mol -1,表明它们对GPER的亲和力很强。傅里叶变换红外光谱和三维荧光表明,GPER 的二级结构在与 PFCA 结合后发生了变化。热力学分析显示 Δ G < 0,这表明 GPER 和 PFCA 之间的相互作用是自发的。对于 PFBA 和 PFOA 与 GPER 的结合,Δ H > 0 和 Δ S > 0,表明相互作用主要由疏水力驱动;对于 PFDoA 与 GPER 的结合,Δ H < 0 和 Δ S< 0,表明范德华力和氢键是主要的相互作用力。分子动力学模拟表明,GPER-PFCA 复合物的稳定性高于游离 GPER,并且 GPER 的结构和疏水性在与 PFCA 结合后发生了变化。分子对接分析表明,三种PFCA都可以与GPER形成氢键,提高了复合物的稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号